Biomarker Testing for Autoimmune Rheumatic Disease - CAM 128
Description
Systemic autoimmune rheumatic diseases (SARDs) are a diverse group of conditions that primarily affect the joints, bones, muscle, and connective tissue.1 SARDs are characterized by dysregulated immunity and inflammatory responses, resulting in damage and destruction to joints, connective tissues, skin, blood elements, and other target organs; however, considerable diversity in clinical presentation, disease course, and treatment response exists.2
The diagnostic workup for SARDs may involve the antinuclear antibody (ANA) assay, which is used to detect autoantibodies (AAB) against intracellular antigens, originally known as antinuclear antibodies.3 Commonly used as part of the initial diagnostic workup to screen for evidence of systemic autoimmunity,4 detection and identification of AABs are important in the diagnosis of SARDs, such as systemic lupus erythematosus (SLE), Sjögren's syndrome (SjS), mixed connective tissue disease (MCTD), systemic sclerosis (SSc), and idiopathic inflammatory myopathies (IIMs).5 Extractable nuclear antigens or ENAs (a historical term from when the antigens were extracted from the cell into saline solution prior to testing) include Sm, U1 ribonucleoprotein (RNP), Ro, and La antigens, and are also useful for evaluating individuals with suspected connective tissue disease.6
Regulatory Status
Food and Drug Administration (FDA)
Many labs have developed specific tests that they must validate and perform in house. These laboratory-developed tests (LDTs) are regulated by the Centers for Medicare & Medicaid Services (CMS) as high-complexity tests under the Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88). LDTs are not approved or cleared by the U.S. Food and Drug Administration; however, FDA clearance or approval is not currently required for clinical use.
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
- For individuals with signs or symptoms of an autoimmune disease, screening for disease using antinuclear antibodies (ANA) is considered MEDICALLY NECESSARY:
- Once during initial workup.
- Up to two additional tests per lifetime if new or more severe signs or symptoms of an autoimmune disease develop.
- For individuals with an abnormal, raised ANA titer and a clinical correlation with the appropriate autoimmune disorder, extractable nuclear antigens (ENA) panel testing of specific autoantibodies is considered MEDICALLY NECESSARY.
- For individuals with painful and swollen joints suggestive of rheumatoid arthritis (RA), testing for rheumatoid factor (RF) and/or anti-cyclic citrullinated peptide (anti-CCP) antibodies is considered MEDICALLY NECESSARY:
- Once during initial workup.
- If initial testing did not result in a diagnosis of RA, up to two additional tests per lifetime if symptoms persist or additional symptoms of RA develop.
- For individuals with an initial positive ANA test and a diagnosis of systemic autoimmune rheumatic disease, testing of dsDNA up to four (4) times per year is considered MEDICALLY NECESSARY.
- For individuals with a negative or low positive ANA test, the following condition specific antibody testing is considered MEDICALLY NECESSARY:
- Testing for anti-Jo-1 in a unique clinical subset of myositis.
- Testing for anti-SSA in the setting of lupus or Sjögren’s syndrome.
- Monitoring of disease with ANA testing or ANA titers is considered NOT MEDICALLY NECESSARY.
- For individuals without symptoms suggestive of an autoimmune disorder, ANA and/or ENA testing is considered NOT MEDICALLY NECESSARY.
- For all other situations not described above, testing of specific antibodies in the absence of a positive ANA test is considered NOT MEDICALLY NECESSARY.
- For asymptomatic individuals, testing of ANA and/or ENA during a wellness visit or a general exam without abnormal findings is considered NOT MEDICALLY NECESSARY.
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of an individual’s illness.
- For the diagnosis of RA, testing for serum biomarkers not discussed above, alone or in a panel (e.g., Seronegative Rheumatoid Arthritis Profile), is considered NOT MEDICALLY NECESSARY.
- For the management of RA, serum biomarker panel testing (e.g., Vectra DA score, PrismRA) is considered NOT MEDICALLY NECESSARY.
- For the diagnosis of systemic lupus erythematosus (SLE), the use of cell-bound complement activation products (e.g., AVISE Lupus) is considered NOT MEDICALLY NECESSARY.
- For the diagnosis, prognosis, or monitoring of SLE or connective tissue diseases, serum biomarker panel testing with proprietary algorithms and/or index scores (e.g., AVISE CTD, AVISE SLE Monitor, AVISE SLE Prognostic, aisle® DX Disease Activity Index, Early Sjögren’s Syndrome Profile) is considered NOT MEDICALLY NECESSARY.
Table of Terminology
| Term |
Definition |
| AAB |
Autoantibodies |
| AAP |
American Academy of Pediatrics |
| ACL |
Anticardiolipin |
| ACP |
American College of Pathologists |
| ACPA |
Anti-citrullinated peptide antibodies |
| ACR |
American College of Rheumatology |
| AIH |
Autoimmune hepatitis |
| AIIF |
Automated indirect immunofluorescence |
| ANA |
Antinuclear antibody |
| Anti La/SS-B |
Anti La/Sjögren Syndrome-B |
| Anti-C1q |
Autoantibodies against C1q |
| Anti-CCP |
Anti-cyclic citrullinated peptide |
| Anti-dsDNA |
Anti-double-stranded DNA |
| Anti-RNP |
Antinuclear ribonucleoprotein |
| Anti-Ro/SS-A |
Anti-Ro/Sjögren Syndrome related antigen A autoantibodies |
| Anti-Sm |
Anti-Smith antibodies |
| APL |
Antiphospholipid antibodies |
| BC4d |
B-lymphocyte-bound C4d |
| BSR |
British Society for Rheumatology |
| CBC |
Complete blood count |
| CB-CAPs |
Cell-bound complement activation products |
| CCP |
Cyclic citrullinated peptides |
| CDC |
Centers for Disease Control and Prevention |
| CENP |
Centromere protein B |
| CIA |
Chemiluminescence immunoassay |
| CLIA ’88 |
Clinical Laboratory Improvement Amendments of 1988 |
| CMS |
Centers for Medicare & Medicaid Services |
| CRP |
C-reactive protein |
| CTD |
Connective tissue diseases |
| CV |
Coefficient of variation |
| ds |
Double-stranded |
| dsDNA |
Double-stranded DNA |
| EC4d |
C4d bound to erythrocytes |
| eGFR |
Estimated glomerular filtration rate |
| EIA |
Enzyme immunoassay |
| ELISA |
Enzyme-linked immunosorbent assay |
| ENA |
Extractable nuclear antigens |
| ESPGHAN |
European Society for Pediatric Gastroenterology Hepatology and Nutrition |
| ESR |
Erythrocyte sedimentation rate |
| EULAR |
European League Against Rheumatism |
| FEIA |
Fluorescence enzyme immunoassay |
| HEp-2 |
Human epithelial type 2 |
| ICAP |
International Consensus on ANA staining Patterns |
| IFA |
Immunofluorescence assay |
| IIF |
Indirect immunofluorescence |
| IIMs |
Idiopathic inflammatory myopathies |
| IQ |
Interquartile |
| ISLM |
Italian Society of Laboratory Medicine |
| JIA |
Juvenile idiopathic arthritis |
| Jo-1 |
Histidyl t-RNA synthetase |
| LAC |
Lupus anticoagulant |
| LDT |
Laboratory developed test |
| LE cell |
Lupus erythematosus cell |
| LFA |
Lupus Foundation of America |
| MAP |
Multianalyte assay panel |
| MCTD |
Mixed connective tissue disease |
| MIA |
Multiplex immunoassay |
| MIIF |
Manual indirect immunofluorescence |
| PC |
Positive concordance |
| PMPM |
Per member per month |
| PPPM |
Per patient per month |
| RA |
Rheumatoid arthritis |
| RF |
Rheumatoid factor |
| RNP |
Ribonucleoprotein |
| SARDs |
Systemic autoimmune rheumatic diseases |
| SDI |
SLICC damage index |
| SDLT |
Standard diagnosis laboratory testing |
| SELENA |
Safety of Estrogens in Lupus National Assessment |
| SjS |
Sjögren's syndrome |
| SLE |
Systemic lupus erythematosus |
| SLICC |
Systemic Lupus International Collaborating Clinics |
| SRDs |
Systemic rheumatic diseases |
| SS-B/La |
Sjögren’s syndrome Type-B |
| SSc |
Systemic sclerosis |
Rationale
Autoimmune diseases occur when an individual’s immune system mistakenly attacks his or her own tissue. This can lead to a variety of conditions and diseases which vary in severity. Autoimmune diseases are estimated to affect five to ten percent of the industrial world population;7 autoimmune conditions are associated with increased morbidity and mortality, and are among the leading causes of death (under 65 years) and disability for women in the US.8
Systemic lupus erythematosus (SLE) is one of more than 80 recognized autoimmune disorders, affecting approximately 204,000 people in the United States.9,10 SLE can present with a wide range of clinical manifestations, typically related to connective-tissue disorders, and often mimics other illnesses.11 This autoimmune disorder leads to inflammation and irreversible damage in one or more organs, including the joints, skin, nervous system, and kidneys.12 The cause of SLE is not entirely understood, but it is predicted to manifest due to a combination of genetic and environmental factors, such as vitamin D deficiency, sunburn, and/or viral infections.13 SLE affects women more than men and is a challenging disease to diagnose because of a broad assortment of signs, symptoms, and serological abnormalities.12 SLE morbidity can be attributed to both tissue damage, toxic treatments, and complications associated with treatments, such as immunosuppression, long-term organ damage due to corticosteroid therapy, and accelerated coronary artery disease.12,14 An early SLE diagnosis is particularly challenging as early-stage tests lack specificity; further, clinical signs and symptoms often only appear after organ damage has occurred, indicating later stages of the disease.15 SLE diagnoses are made based on lab findings, clinical manifestations, serology, and histology of impacted organs.15 However, current SLE screening tests are notoriously unreliable.16
Rheumatoid arthritis (RA) affects more than one million adults in the United States. RA is characterized by chronic inflammation of the synovial tissue of joints, cartilage, and bone.17-21 Pathological abnormalities in patients with RA includes chronic synovitis, which results in joint devastation.17,18,20 Cellular and humoral response aberrations result in autoimmunity; antibodies and rheumatoid factors against post-translational modified proteins (including modifications such as citrullination). As such, synthetic cyclic citrullinated peptides (CCP) have been developed for diagnostic use.20
There is consensus to the value of serological testing for diagnostic purposes: both rheumatoid factor (RF) and anti-citrullinated peptide antibodies (ACPA) tests have diagnostic value in patients suspected of having RA (but not in asymptomatic patients as a general screen).22 Diagnostic testing with RF should be restricted to those with a moderate to high pretest probability of rheumatoid arthritis. RF testing should not occur in patients with joint pain in the absence of synovitis (e.g., nonspecific arthralgias, fibromyalgia, OA) because a positive test result is more likely to represent a false-positive result. ACPA testing is useful as a diagnostic test in patients with a moderate to high pretest probability of rheumatoid arthritis, but similarly, should not be used in those with a low pre-test probability. For patients “with an inflammatory, small joint arthritis and with a moderate to high pretest probability of RA, the presence of ACPA testing confirms a diagnosis of RA.”22
To date, the etiology of RA has not been fully elucidated, though recent studies have suggested that genetic, epigenetic, and environmental factors contribute to RA presentation.17,20 Due to the complexity of RA pathogenesis, there is no model drug to cure RA.
Biologic markers or “biomarkers” can provide objective measurements that reflect underlying pathophysiological processes, pathogenic processes, or responses to treatment. Most measures of monitoring disease and treatment progress rely on subjective measurements, such as joint evaluation, so biomarkers may be a useful complement in patient management.23 Joint damage at the molecular level may be occurring before any clinical signs appear so identifying any indications of disease activity could allow clinical interventions to be taken earlier.24 Markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are part of clinical measures such as the DAS. However, these two biomarkers are nonspecific; abnormal amounts
of these markers may be due to other reasons apart from RA and may be completely normal in patients with RA.25,26 This non-specificity is not limited to ESR and CRP. For example, antibodies (usually called rheumatoid factors or RF) produced against immunoglobulin G (IgG) are often tested to diagnose RA, but these antibodies may be produced in response to another rheumatic condition or a separate chronic infection.27 Autoantibodies to citrullinated protein epitopes, such anti-cyclic citrullinated peptide (anti-CCP2), has also been a focus of biomarker research in RA. Both RF and anti-CCP2 have similar sensitivities for the diagnosis of RA, but anti-CCP2 is positive in 20%-30% of RA patients who are negative for RF.28 RA is a heterogenous condition, and no single biomarker is a reliable predictor of RA disease activity.24
Currently, conventional synthetic disease modifying anti-rheumatic drugs (csDMARDs) are the first line of RA therapy. Unfortunately, some RA patients do not respond to csDMARDs and clinical guidelines suggest use of alternative therapies such as biologic DMARDs (bDMARDs). bDMARDs are more specific to inflammatory factors than csDMARDs and more efficient in demonstrating remission and inducing low disease activity.29 Several bDMARDs are available for RA management, and these include TNFis.17-19,30 TNFi treatment, however, is not without limitations. Unfortunately, the majority of patients fail to respond to TNFi treatment (measured by American College of Rheumatology (ACR)50-indicates 50% disease improvement) and only 10-25% achieve remission.17,19,21,31 Currently, there is no way to predict whether RA patients will respond to TNFi therapy, and approximately three months is needed to determine whether a patient is responding.17,19 Accordingly, there has been a push to create a personalized medicine approach to identify non-responders to enhance clinical outcomes.17,19
The systems by which the immune system maintains tolerance to an individual's own antigens can be overcome by release of intracellular antigens following excessive cell death, ineffective clearance of apoptotic debris, inflammation-induced modification of self-antigens, or molecular mimicry, leading to the production of antibodies against self-antigens or autoantibodies (AAB).32 Autoantibodies mediate both systemic inflammation and tissue injury and may play a role in the pathogenesis of many autoimmune diseases.32 Generally, AAB development precedes the clinical onset of autoimmune disease and has predictive value;4 thus, AABs serve as good serological markers to screen for evidence of autoimmunity.33,34 Autoantibodies can target a variety of molecules (including nucleic acids, lipids, and proteins) from many cellular localizations—nucleus, cytoplasm, cell surface, extracellular organelles,32 and different specific AABs are associated with particular diagnoses, symptoms, unique syndromes, subsets of disease, and clinical activity.4 See Table 1 from Suurmond and Diamond (2015) below:
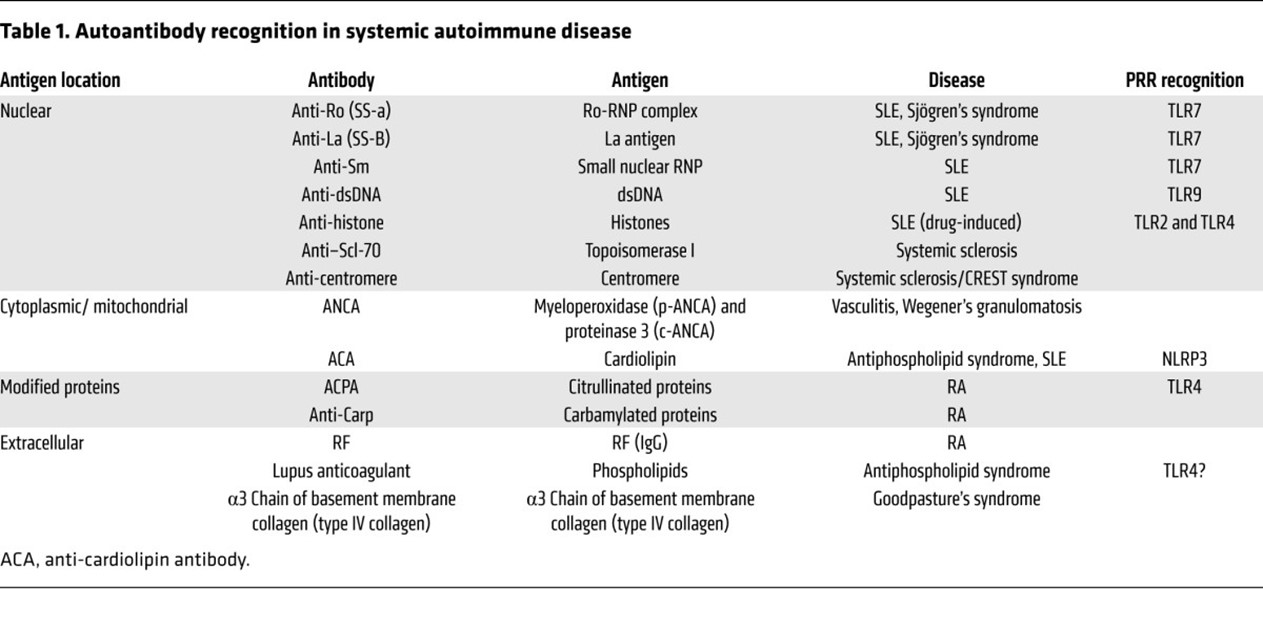
However, serum AAB are present in 18.1% of the general population, and titers are higher in females and increase with age.35 Additionally, only in a few cases does the antibody titer correlates with the severity of clinical manifestations or the response to treatment.34 The use of ANA detection as a diagnostic test originated with the observation of the lupus erythematosus (LE) cell.36 Since then, several tests have been developed to detect these antibodies.
The indirect immunofluorescence (IIF) test is the most widely used assay for the detection of AAB and remains the reference method of choice.37 Detection of ANAs by the IIF technique demonstrates binding to specific intracellular structures within the cells, resulting in staining patterns reported using the consensus nomenclature and representative patterns defined by The International Consensus on ANA staining Patterns (ICAP) initiative and the degree of binding reflected by the fluorescence intensity or titer.5,38 The test takes advantage of a HEp-2 cell line, which have large, easy to visualize, nuclei and contain nearly all of the clinically important autoantigens, making these cells ideal for the detection of the corresponding AABs.39 The ANA IIF assay using HEp-2 slide has a high sensitivity for screening of SARDs and efforts to harmonize the nomenclatures for testing and reporting have made this a powerful screening tool.5 The frequency of ANA in SLE and SSc is 95–100%, 50–70% in SJS and 30–50% in rheumatoid arthritis (RA):4 however, their isolated finding in an otherwise healthy individual has a low positive predictive value which needs to be integrated with other laboratory parameters and patient risk factors.35 Disadvantages of the indirect immunofluorescence test include its labor-intensiveness, significant training requirements for competence, and subjectivity in titer and pattern recognition; moreover, because the staining pattern usually does not identify the responsible autoantibody, additional testing may be required.5,39 Automated image analysis provides a viable option for distinguishing between positive and negative results although the ability to assign specific patterns is insufficient to replace manual microscopic interpretation.40
The antinuclear antibody (ANA) test is commonly used in the evaluation of autoimmune disorders, as these antibodies are responsible for attacking healthy or normal cells. More than 95% of individuals with SLE will have a positive ANA test.16 However, ANAs are present in “a significant proportion of normal individuals and lacks specificity or prognostic value.”15 In particular, approximately only 11-13% of individuals with a positive ANA test will actually have SLE, and approximately 15% will be completely healthy.16 Other SLE diagnostic methods include the monitoring of anti-double-stranded DNA (anti-dsDNA), C3 and C4 complement levels, CH50 complement levels, erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) levels, antiphospholipid antibodies, and urine protein-to-creatinine ratios.41
If SLE is suspected based on the clinical picture following a positive ANA screen, the sera should be tested for antibodies to double-stranded DNA (dsDNA). Anti-dsDNA antibodies are present in two-thirds of patients with SLE, and they have a good association with disease activity and lupus nephritis. Serial monitoring of anti-dsDNA antibodies has modest correlation with disease activity.33
A positive ANA screen should also be followed by identification of sub-specificities by screening for antibodies to extractable nuclear antigens (ENAs). ENAs were identified by using saline extract of nuclei as the antigen. Antibodies to ENA can be determined using double immunodiffusion, immunoblotting, enzyme-linked immunosorbent assays (ELISA), or bead-based assay using recombinant or affinity-purified antigens. Different ENAs have an association with different connective tissue diseases.33
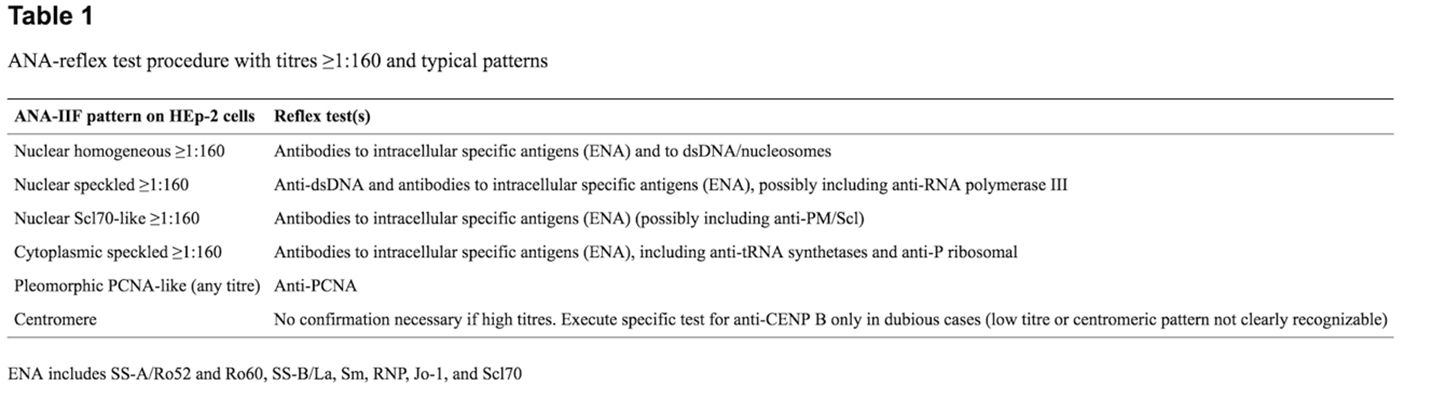
Reflex tests for positive ANA screens have been proposed to improve appropriateness in diagnosis of SARDs and avoid unnecessary second level testing. For specific autoantibodies responsible for certain fluorescent ANA patterns, such as homogeneous, speckled, fine grainy (Scl70-like), nucleolar, centromeric or speckled cytoplasmic, the identification of precise autoantibody markers is considered essential while for others it is not deemed to be necessary.42 See Table 1 from Tonutti, et al. (2016), below.
Proprietary Testing
A set of proprietary tests are available from Exagen, under the “AVISE” line. Their line of tests utilizes a two-tiered testing method and a novel algorithm that measures 10 SLE relevant markers to deliver an index calculation value suggestive of the presence or absence of SLE. This includes tests for prognosis (10 biomarkers including various autoantibodies such as anti-C1q and antiribosomal P), diagnosis (10 biomarkers, includes ENA panel), and monitoring (six biomarkers, includes anti-dsDNA and anti-C1q). AVISE CTD (standing for connective tissue disease) is intended to assist with the differential diagnosis of several autoimmune diseases and includes several ANA biomarkers, as well as an ENA panel. Other tests offered, such as AVISE Anti-CarP (evaluates autoantibodies to carbamylated proteins for rheumatoid patients) still include ANA components.43
AVISE Lupus by Exagen is a laboratory developed test (LDT) designed to assist in SLE diagnoses. This LDT utilizes a two-tiered testing method and a novel algorithm that measures 10 SLE relevant markers to deliver an index calculation value suggestive of the presence or absence
of SLE. The AVISE Lupus test also uses cell-bound complement activation products (CB-CAPs) to measure complement system activation.44 The 10 SLE relevant markers in this test include anti-dsDNA, anti-Smith (anti-Sm) antibodies, erythrocyte-bound C4d or B-lymphocyte-bound C4d (BC4d), ANA, CB-CAPs, and autoantibody specificity components.44 As noted on their website, “The AVISE Lupus test is an ideal test for ANA positive patients with a clinical suspicion of lupus.”44
PrismRA is a molecular signature test that predicts TNFi non-response prior to treatment initiation. PrismRA utilizes a 23-feature blood-based molecular signature response classifier (MSRC) which integrates next generation RNA sequencing data and clinical features (clinical metrics, demographic variables, C reactive protein (CRP) and anti-CCP antibodies) to predict patients’ response to TNFi treatment.21 A high score is indicative of decreased likelihood of the RA patient to respond to TNFi therapies.
Vectra DA is a multi-biomarker disease activity (MBDA) blood test which combines the levels of 12 serum biomarkers into a single score from 1 to 100 to provide an objective measure of RA disease activity. It is intended for use with existing symptom-based disease activity measures to improve long-term outcomes for RA patients.45 While multi-biomarker panels are emerging as a potentially useful tool in the management of RA, there is not yet a consensus as to their clinical utility.23
The proprietary test, aiSLE DX Disease Activity Index, is a blood test that measures the level of disease activity and supports clinicians in the measurement of lupus (SLE) and the assessment of treatments. This test examines a well-defined group of immune modulatory soluble mediators, such as cytokines, chemokines, and soluble receptors, which have been linked to disease activity in plasma. The panel of immune mediators assessed in this test are able to differentiates individuals with active clinical illness from those with low activity or quiescent disease in a simple blood test.46
Seronegative Rheumatoid Arthritis Profile is a blood test which includes Rheumatoid Factor (RF) IgG, IgA, IgM, Antibodies to Cyclic Citrullinated Peptide (CCP)- IgG, and Scavenger Receptor A (SR-A) which helps to identify antinuclear antibodies. This test is seen as a more robust serological diagnostic panel for RA.47
The Early Sjögren’s Syndrome Profile from Immco Diagnostics Inc. is a blood test that combines traditional and novel markers for early diagnosis with higher sensitivity and specificity. These novel markers include SP-1, CA-6, and PSP which helps to increase the sensitivity to over 80% and enables diagnosis before the traditional markers, SS-A (Ro) and SS-B (La), appear.48
Analytical Validity
A variety of manual or automated single or multiplex immunoassays have been introduced to make the process of detecting autoantibodies more efficient, including ELISA, fluorescent microsphere assays, and chemiluminescence immunoassays (CIA)—each with different performance characteristics.5 In these assays, a panel of purified native or recombinant autoantigens is prepared, and each antigen is immobilized on a solid surface (microtiter plate, fluorescent microsphere, or membrane) and incubated with diluted human serum.39 The advantages of these alternative approaches to ANA IIF testing include their suitability for high-throughput testing, semi-quantification of test results, the lack of subjectivity, and the consolidation of ANA-related tests in a single platform as a positive test also provides identification of the responsible autoantibody.5,39 It has been estimated that solid phase assays may decrease the labor cost of ANA testing by as much as 95%.39 In a recent study which evaluated the performance of an automated CIA and fluorescence enzyme immunoassay (FEIA) and compared their performance to that of IIFA, both FEIA and CIA screen significantly outperformed IIF, with a higher specificity for FEIA and higher sensitivity for CIA.49 The use of solid phase assays as the initial test for the detection of ANA is concerning because the number of autoantigens that are included in solid phase assays is limited compared with the number that are present in the Hep-2 cell substrate, thus limiting sensitivity.39 Consequently, IIF remains the gold standard, and in cases of strong clinical suspicion of SARD and a negative screen from a solid phase assay, IIF should be performed.49
Tipu and Bashir (2018) investigated the specificity and pattern for ANA in systemic rheumatic disease patients. A total of 4347 samples were sent, and 397 were positive for ANA. Of these 397, 96 were positive on the anti-ENA screen and tested for anti-ENA reactivity. Anti-SSA antibodies were found in 59 of these samples. The most common ANA patterns were “coarse” and “fine-speckled” (43 and 22 of 81 respectively). However, no specific ANA pattern was associated with anti-ENA reactivity.50
Kim et al. (2019) performed a meta-analysis comparing ANA measurement by automated indirect immunofluorescence (AIIF) and manual indirect immunofluorescence (MIIF). A total of 22 studies including 6913 positive and 1818 negative samples of manual indirect immunofluorescence (MIIF) were included. Among this cohort, 524 samples with combined systemic rheumatic diseases (SRDs), 132 systemic lupus erythematosus (SLE) samples, and 104 systemic sclerosis (SSc) samples, and 520 controls were available. Positive concordance (PC) between AIIF and MIIF was 93.7%, although PC of total pattern and titer were lower. Clinical sensitivities of AIIF vs MIIF were 84.7% vs 78.2% for combined SRDs, 95.5% vs 93.9% for SLE, and 86.5% vs 83.7% for SSc. Clinical specificities of AIIF vs MIIF were 75.6% vs 79.6% for combined SRDs, 74.2% vs 83.3% for SLE, and 74.2% vs 83.3% for SSc. The authors concluded that the sensitivities did not differ between methods, but the specificities of SLE and SSc were statistically significant changes.51
Dervieux, et al. (2017) performed the analytical validation of Exagen’s multianalyte panel test for SLE. This assay uses quantitative flow cytometry to assess the levels of the complement split product C4d bound to erythrocytes (EC4d) and B-lymphocytes (BC4d), in units of mean fluorescence intensity (MFI), and immunoassays to assay for antinuclear and anti-double stranded DNA antibodies (e.g. autoantibodies). The results were reported on a two-tiered index score as either positive or negative. The authors included specimens from both patients with SLE as well as individuals without SLE. Controls consisting of three-level C4 coated positive beads were run daily. The authors note that at ambient temperature both EC4d and BC4d are stable for two days and for four days if the samples are stored at four degrees Celsius. “Median intra-day and inter-day CV [coefficient of variation] range from 2.9% to 7.8% (n=30) and 7.3% to 12.4% (n=66), respectively. The 2-tiered index score is reproducible over 4 consecutive days upon storage of blood at 4°C. A total of 2,888 three-level quality control data were collected from six flow cytometers with an overall failure rate below 3%. Median EC4d level is six net MFI (Interquartile [IQ] range 4-9 net MFI) and median BC4d is 18 net MFI (IQ range 13-27 net MFI) among 86,852 specimens submitted for testing. The incidence of 2-tiered positive test results is 13.4%.”52
Putterman, et al. (2014) compared the performance of C4d CB-CAPs on erythrocyte and B cells with antibodies to dsDNA, C3, and C4 in patients with SLE. A total of 794 individuals participated in this study, which included 205 healthy controls, 304 patients with SLE, and 285 patients with other rheumatic diseases. Both erythrocytes and B cells were measured with flow cytometry, and antibodies, including anti-dsDNA, were measured with solid-phase immunoassays. SLE activity was determined using the SLE Disease Activity Index Safety of Estrogens in Lupus National Assessment (SELENA) Modification, and the two-tiered AVISE Lupus test was developed. Results showed that “The combination of EC4d and BC4d in multivariate testing methodology with anti-dsDNA and autoantibodies to cellular and citrullinated antigens yielded 80% sensitivity for SLE and specificity ranging from 70% (Sjogren’s syndrome) to 92% (rheumatoid arthritis) (98%vs. normal).”53 Overall, the measurement of CB-CAPs was more sensitive for SLE diagnostic purposes than complement or anti-dsDNA measurements.
Ramsey-Goldman, et al. (2020) evaluated the use of CB-CAPs, using flow cytometry, or a multianalyte assay panel (MAP) that includes CB-CAPs (e.g., AVISE Lupus) on patients with suspected SLE (n = 92) who fulfilled three classification criteria of the American College of Rheumatology (ACR). They also compared the data with individuals with established SLE (n = 53). At the initial visit, the individuals with suspected SLE had statistically higher positive CB-CAP (28%) or MAP results (40%) than individuals with established SLE. “In probable SLE, MAP scores of >0.8 at enrollment predicted fulfillment of a fourth ACR criterion within 18 months (hazard ratio 3.11, P<0.01).” The authors, who did acknowledge compensation from Exagen, conclude that “[a] MAP score above 0.8 predicts transition to classifiable SLE according to ACR criteria.”54
Clinical Utility and Validity
ANA, ENA, and SDLT
Oglesby, et al. (2014) performed a cost-savings impact analysis on when the diagnosis of SLE is made and how it affects the clinical and economic outcomes. Using a claims database of claims made between January 2000 and June 2010, the authors separated individuals into two groups (n = 4166 per group) —early diagnosis (within six months of onset of symptoms) and late diagnosis (6 or more months after the onset of symptoms)—based upon an algorithm using a patient’s ICD-9 diagnosis code(s) on the claim(s) and when SLE medications were dispensed. Additional propensity scores were matched using data based on “age, gender, diagnosis year, region, health plan type, and comorbidities.” Results show that the early diagnosis group had lower rates of mild, moderate, and severe flares as well as lower rates of hospitalization as compared to the late diagnosis group. Moreover, “[c]ompared with the late diagnosis patients, mean all-cause inpatient costs PPPM [per patient per month] were lower for the early diagnosis patients (US$406 vs. US$486; p = 0.016). Corresponding SLE-related hospitalization costs were also lower for early compared with late diagnosis patients (US$71 vs US$95; p = 0.013).” The values are adjusted to 2010 US dollars. The authors note that the other resource use and cost categories were consistent, concluding “[p]atients diagnosed with SLE sooner may experience lower flare rates, less healthcare utilization, and lower costs from a commercially insured population perspective.”55
A study by Yeo, et al. (2020) demonstrates that there is little benefit to repeat ANA testing if the initial test was negative by evaluating the cost of repeat ANA testing. From 2011 to 2018, 36,715 ANA tests were performed for 28,840 patients at a total cost of $675,029. Of these tests, 21.4% were repeats in which 54.9% of the patients initially tested negative. Of those who tested negative and repeated ANA testing, only 19% of the patients had a positive result when the test was repeated once in under two years, and this positive test did not lead to a change in diagnosis. Therefore, the authors conclude that “repeat ANA testing after a negative result has low utility and results in high cost.”56
Deng, et al. (2016) investigated the clinical utility of ANA testing through different assays to see which one was most appropriate for evaluating patients with CTD. With 1000 samples collected, they compared an enzyme immunoassay (EIA), immunofluorescence assay (IFA), and multiplex immunoassay (MIA) in terms of specificity and sensitivity of testing. The researchers found that through using weights to define a patient sample that reflected the intended testing population and a normalized specificity of 90% to standardize the comparison between tests, the MIA, EIA, and IFA had sensitivities of 67%, 67%, and 56%, respectively. However, with a varying clinical cutoff, the IFA could obtain a sensitivity of 94% and a corresponding specificity of only 43%. This demonstrated that the sensitivity and specificity could easily vary with predetermined cutoffs; but, there were “no statistically significant differences in the clinical utility of the IFA, EIA, or MIA.”57
Alsaed, et al. (2021) compared the performance of ANA testing via ELISA vs IIF for CTDs. From a sample of 1457 patients and 12,439 tests ordered in 2016, they found that with “cut-off ratio ≥ 1.0 for ANA-ELISA and a dilutional titre ≥ 1:80 for ANA-IIF, the sensitivity of ANA-IIF and ANA-ELISA for all CTDs were 63.3% vs 74.8% respectively. For the SLE it was 64.3% vs 76.9%, Sjogren's Syndrome was 50% vs 76.9% respectively. The overall specificity of ANA-ELISA was 89.05%, which was slightly better than ANA-IIF 86.72%.” This communicated the ELISA was slightly better than IIF in sensitivity and specificity, which could influence the convention of using IIF going forward if these findings are reflected in other cohort studies.58
Biomarker analysis
Wallace, et al. (2019) performed a randomized prospective trial to assess the clinical utility of the AVISE lupus MAP test (MAP/CB-CAP) as compared to standard diagnosis laboratory testing (SDLT). A total of 145 patients with a history of positive antinuclear antibody status were randomly assigned to either an SDLT arm (n = 73) or the MAP/CB-CAP arm (n = 72) of the study. Treatment changes were recorded based on either the SDLT or MAP/CB-CAP results. Even though the demographics between the two arms of the study were similar, the results were different. “Post-test likelihood of SLE resulting from randomisation in the MAP/CB-CAPs testing arm was significantly lower than that resulting from randomisation to SDLT arm on review of test results (−0.44±0.10 points vs −0.19±0.07 points) and at the 12-week follow-up visit (−0.61±0.10 points vs −0.31±0.10 points) (p<0.05). Among patients randomised to the MAP/CB-CAPs testing arm, two-tiered positive test results associated significantly with initiation of prednisone (p=0.034).”59 The authors conclude that testing such as the AVISE Lupus test has clinical utility and does affect treatment decisions.
A longitudinal, retrospective study by Mossell, et al. (2016) of 46 patients who were anti-nuclear antibodies (ANA) positive but SLE-specific autoantibodies negative was conducted to evaluate the clinical utility of the AVISE Lupus test. There was a total of 23 patients in the “case” group (i.e. positive result based on the AVISE Lupus test), and 23 patients were in the “control” or negative results group. The charts of each individual were reviewed at two different times: T0 (or the initial time) and T1 (or approximately one year later). The case group was diagnosed with SLE at a higher rate than the control group (87% vs. 17%, respectively); moreover, the case group fulfilled four of the ACR classification criteria of SLE at a higher rate than the control group (43% vs 17%, respectively). The authors found that the sensitivity of the AVISE Lupus test (83%) is statistically significantly higher than the ACR score (42%, p = 0.006). Even at the initial baseline, individuals in the case group were prescribed anti-rheumatic medications more frequently (83% vs. 35%, p = 0.002) than the control group, indicating that a positive AVISE Lupus test may result in a more aggressive early treatment therapy.60
Liang, et al. (2020) assayed the utility of the AVISE test in predicting lupus diagnosis and progression in 117 patients who previously did not have a diagnosis of SLE. The study assessed the patients at the time of the initial AVISE test (t = 0) and two years later (t = 2) using the SLE diagnosis criteria of the Systemic Lupus International Collaborating Clinics (SLICC) and ACR and the SLICC Damage Index (SDI) to measure SLE damage. After two years, patients who tested positive developed SLE at a significantly higher rate than those who tested negative using the AVISE test (65% vs 10.3%, p < 0.0001). AVISE-positive patients have more SLE damage after two years than AVISE-negative patients (1.9±1.3 vs 1.03±1.3, p=0.01). In particular, the authors note that the levels of BC4d “correlated with the number of SLICC criteria at t=0 (r=0.33, p< 0.0001) and t=2 (r=0.34, p<0.0001), as well as SDI at t=0 (r=0.25, p=0.003) and t=2 (r=0.26, p=0.002).”61
Alexander, et al. (2021) further validated the clinical utility of the AVISE lupus test via a systematic review of medical records of ANA-positive patients with positive (>0.1) or negative (<-0.1) MAP scores. They found that the “odds of higher confidence in SLE diagnosis increased by 1.74-fold for every unit increase of the MAP score” with statistical significance, demonstrating that the test still further solidifies a diagnosis of SLE and can help inform “appropriate treatment decisions.”62
A study by Clarke, et al. (2020) demonstrates the cost-effective management of systemic lupus erythematosus (SLE) using a MAP rather than SDLTs. The higher specificity of MAP allows for an earlier SLE diagnosis, prompt initiation of the appropriate therapy, and fewer unnecessary and costly hospitalizations or investigations. Current SDLTS, such as ANA tests, have a high diagnostic sensitivity, but a high false-positive rate. MAP combines complement C4d activation products on erythrocytes and B cells with SDLTs, with antibodies to nuclear antigens, dsDNA IgG (with Crithidia confirmation), Smith, Sjogren’s syndrome type-B (SS-B/La), topoisomerase I (Scl-70), centromere protein B (CENP), histidyl t-RNA synthetase (Jo-1), and cyclic citrullinated peptites (CCP) to improve SLE diagnosis. MAP “yields improved overall diagnostic performance with a sensitivity and specificity of 80% and 86%, respectively, compared with a sensitivity and specificity of 83% and 76%, respectively, for SDLTs. Despite the lower sensitivity, the superior specificity of MAP (86%) over SDLTs (76%) results in a higher positive predictive value associated with MAP (36.75%) compared with SDLTs (26.02%).”63 The improved specificity of MAP resulted in a cost savings of $1,991,152 to a US commercial plan over a four year time horizon, which translates to $0.04 in per member per month (PMPM) savings.63
Clinical validation of PrismRA was conducted in the Comparative Effectiveness Registry to Study Therapies for Arthritis and Inflammatory Conditions (CERTAIN) study.30,64 The CERTAIN trial was conducted by the Consortium of Rheumatology Researchers of North America which consisted of 43 sites and 117 rheumatologists.64 This prospective study analyzed baseline RNA sequencing and clinical assessments to determine the effectiveness of PrismRA to predict TNFi non-response. Evaluation of the clinical response to TNFi was performed at six months and was determined by ACR50. The CERTAIN study built and validated the biomarker panel used for MSRC analyses. The study found that PrismRA demonstrated a positive predictive value of 89.7%, a specificity of 86.8%, and a sensitivity of 50%.19,64
Inadequate TNFi response predictions were further validated on integrated blood samples from CERTAIN and NETWORK-004 studies. NETWORK-004 was a 24-week blinded prospective study conducted at 73 sites to evaluate the ability of MSRC to identify TNFi non-responders at three and six months by ACR50 (evaluations were also conducted using other scales such as Disease Activity Score (DAS28)-CRP, and Clinical Disease Activity Index). CERTAIN samples were used for transcript biomarker feature selection (n=100) and cross validation of MSRC (n=245). In the NETWORK-004 cohort, MSRC validation was performed in samples from naïve (n=146) and TNFi exposed (n=113) patients. ACR50 of patients stratified by MSRC at six months according to prediction of an inadequate response to TNFi therapy had an odds ratio of 4.1 (95% CI 2.0–8.3; p value=0.0001). Patients with a non-response MSCR were 26 times less likely to achieve remission evaluated three months after TNFi therapy.21 Both studies found that PrismRA was able to accurately predict TNFi non-responders according to multiple clinically validated measurement scales.21,64
Bergman, et al. (2020) performed modeling of the projected improvements from PrismRA and determined that ACR50 improved in the stratified cohort (40%) compared to the unstratified patient cohort (30%) and decreased costs of ineffective treatment by 19%. Further, PrismRA was shown to be a better predictor of inadequate response to TNFi treatment than clinical metrics alone.30 Pappas, et al. (2021) conducted a 32-question decision-impact survey involving 248 rheumatologists to determine whether predictive tests such as PrismRA appear to have clinical utility in RA patients’ ability to respond to TNFi therapy. The study demonstrated that rheumatologists overwhelmingly supported the clinical need of predictive technologies to determine whether RA patients would respond to TNFi therapies and that payers should provide coverage of predictive technology.19
According to Curtis, et al. (2012), the MBDA algorithm (Vectra DA) was developed by screening 396 candidate biomarkers. An algorithm was then created to generate a composite score based on the 12 biomarkers most correlated to RA clinical disease activity which are as follows:
- Interleukin-6 [IL-6]
- Tumor necrosis factor receptor type I [TNFRI]
- Vascular cell adhesion molecule 1 [VCAM-1]
- Epidermal growth factor [EGF]
- Vascular endothelial growth factor A [VEGF-A]
- YKL-40
- Matrix metalloproteinase 1 [MMP-1]
- MMP-3
- CRP
- Serum amyloid A [SAA]
- Leptin
- Resistin
These biomarkers represent several processes related to RA, such as cartilage remodeling and cytokine signaling pathways. A score of ≤29 is considered “low” activity, between 29 and 44 is “moderate” activity, and >44 is “high” activity. The MBDA is intended to provide separate information from a clinical evaluation of joints and should be used as a complement, not as a replacement.25
This MBDA has been shown to correlate significantly (r=0.72; p<0.001) with a disease activity score based on the 28-joint Disease Activity Score based on CRP (DAS28-CRP) and has been validated for clinical use as a disease activity marker in RA.25 Both Hirata, et al. (2013) and Bakker, et al. (2012) found the MBDA score to correlate well with disease activity and could complement other existing measures of RA assessment. Remission based on the MBDA score was a significant predictor of radiographic non-progression, whereas both remission-defined DAS28-CRP and American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) criteria was not. The MBDA test was also useful in assessing the risk of radiographic progression among patients who met clinical remission criteria. MBDA results may provide an important addition to clinical assessment, however, further studies are needed to confirm its clinical utility in the management of RA.45
Li, et al. (2013) evaluated the impact of an MBDA blood test for rheumatoid arthritis (RA) on treatment decisions made by six health care providers (HCPs) in 101 patients. HCPs completed surveys before and after viewing the MBDA test result, recording dosage and frequency for all RA medications and assessment of disease activity. Frequency and changes in treatment plan that resulted from viewing the MBDA test result were determined. The MBDA test results were found to have changed 38% of patients’ treatment plans. Furthermore, treatment plans were changed 63% of the time the MBDA test results were found to be “not consistent” or “somewhat consistent” with the clinical assessment of disease activity. However, any improvement in clinical outcomes caused was not reported, and the overall amount of drug use was not affected.67
Another study by Li, et al. (2016) assessed the correlation between MBDA score and disease progression in 163 RA patients. The study found that low radiographic progression was associated with low MBDA scores, and higher scores were associated with more frequent and severe progression. Notably, MBDA scores correlated with progression even when a conventional measure such as the DAS28 indicated otherwise. For example, low risk of progression was associated with a low MBDA score, even when a concurrent DAS28 score was high. The authors concluded that MBDA may be a good complement for conventional measures, as well as provide information on changing treatment plans.68
Curtis, et al. (2018) initially studied the influence of age, obesity, and other comorbidities on the MBDA test. A cross-sectional analysis of RA patients who have participated in an MBDA test was used (n=357). “Of 357 eligible patients, 76% (n = 273) had normal CRP (<10mg/L) with high (33%), moderate (45%), and low (22%) disease activity by MBDA. The MBDA score was significantly associated with BMI, age, CDAI [clinical disease activity index], and SJC [swollen joint count].”69 Almost one third of participants had normal CRP scores but high MBDA scores. “In this real-world analysis, the MBDA score was associated with RA disease activity, obesity, and age, and was negligibly affected by common comorbidities.”69 The authors conclude by suggesting that an adjusted MBDA score may require development to account for BMI and age. Such a study was then published the following year. Curtis, et al. (2019) developed an MBDA test that will include additional factors such as sex, age and obesity in RA patients. Obesity, or adiposity, was measured using either BMI or serum leptin concentration. Two cohorts were studied, totaling 1736 patients. Overall, the authors have developed “a leptin-adjusted MBDA score that has significantly improved [the] ability to predict clinical disease activity and radiographic progression.”70 It was suggested that this leptin-adjusted MBDA score “significantly adds information to DAS28-CRP and the original MBDA score in predicting radiographic progression. It may offer improved clinical utility for personalized management of RA.”70
A recent study analyzed the measurement of serum biomarkers at early RA disease onset in hopes to better predict disease progression.71 MBDA score and changes in this score were evaluated to predict DAS28-CRP remission. A total of 180 patients participated in this study and were treated with either methotrexate and adalimumab (n = 89) or methotrexate and placebo (n = 91) in addition to a glucocorticoid injection into swollen joints; results showed that “Early changes in MBDA score were associated with clinical remission based on DAS28-CRP at 6 months.”71
In a study by Ma, et al. (2020), the MBDA test was used to explore the role of biomarkers in predicting remission of RA. Serum samples for 148 patients were assessed for MBDA score at three months, six months, and at one year. RA patients on greater than six months stable therapy in stable low disease activity were assessed every three months for one year. Patients not fulfilling any remission criteria at baseline were classified as ‘low disease activity state’ (LDAS). Patients not fulfilling any remission criteria over one year were classified as ‘persistent disease activity’ (PDA). Of the 148 patients, 27% were in the LDAS group and over one year and 9% of patients were classified as PDA. Baseline MBDA score and concentrations of IL-6, leptin, SAA and CRP were significantly lower in all baseline remission criteria groups in comparison to LDAS groups. The individual MBDA biomarkers (IL-6, leptin, SAA, CRP) and initial MBDA score was able to differentiate between remission at baseline and LDAS. The authors state that these findings highlight the potential value of repeated measurements of MBDA score to evaluate the stability of clinical disease activity over time.72
In a combined analysis of the OPERA, SWEFOT, and BRASS studies in which a newer version of the MBDA score was validated, Curtis analyzed the prognostic value of the adjusted MBDA score for radiographic progression in RA. The new MBDA score, used in these three studies, adjusts for age, sex, and adiposity. Curtis evaluated associations of radiographic progression (ΔTSS) per year with the adjusted MBDA score, seropositivity, and clinical measures using linear and logistic regression. The adjusted MBDA score was validated in SWEFOT, compared with the other two cohorts, and used to generate curves for predicting risk of radiographic progression. The adjusted MBDA score was found to be the “strongest, independent predicator of radiographic progression (ΔTSS > 5) compared with seropositivity (rheumatoid factor and/or anti-CCP), baseline TSS, DAS28-CRP, CRP SJC, or CDAI. Its prognostic ability is not significantly improved by the addition of DAS28-CRP, CRP, SJC, or CDAI.”73
Fleischmann, et al. (2022) engaged in a multicenter, randomized, placebo-controlled trial of repository corticotropin injection (RCI) in patients with active RA. The utility of an MBDA score was measured against the utility of the Disease Activity Score to assess disease activity in RA. Study participants received 80 units of RCI twice weekly, and those who had low disease activity at week 12 were given either 80 units of RIC or a placebo twice weekly. The changes in disease activity (measured by DAS28-ESR, CDAI, and MBDA scores) were analyzed, including correlations between MBDA scores and both DAS28-ESR and CDAI scores. Results showed “changes from baseline in DASw8-ESR and CDAI scores suggested the RCI therapy led to clinically meaningful improvements in disease activity, but improvements from baseline in MBDA scores were below the minimally important difference threshold.” The authors concluded that MBDA scores were not “sufficiently responsive” in the assessment of RA disease activity. The authors also said that MBDA should not be used as a preferred disease activity measure for RA patients.74
American College of Rheumatology
Systemic Lupus Erythematosus (SLE)
In 1997, the Diagnostic and Therapeutic Criteria Committee of the ACR revised the 1982 criteria for SLE. Often referred to as the 1997 ACR criteria, these revisions included the addition of “[p]ositive finding of antiphospholipid antibodies based on 1) an abnormal serum level of IgG or IgM anticardiolipin antibodies, 2) a positive test result for lupus anticoagulant using a standard method, or 3) a false-positive serologic test for syphilis known to be positive for at least six months and confirmed by Treponema pallidum immobilization or fluorescent treponemal antibody absorption test.”75 The 1997 ACR criteria consists of 11 possible different criterion and each criterion may have more than one definition. A minimum score of four out of 11 is indicative of SLE. According to the Centers for Disease Control and Prevention (CDC), rheumatologists can use these criteria “to classify SLE for research purposes.”76 The 1997 ACR criteria in a study by Mosca, et al. (2019), using a cohort of 616 patients, has a reported accuracy of 75.5%, sensitivity of 66.1%, and specificity of 91.6%. The criteria are as follows:76,78
- Malar Rash
- Discoid Rash
- Photosensitivity
- Oral Ulcers
- Nonerosive Arthritis
- Pleuritis or Pericarditis
- Renal Disorder
- Neurologic Disorder
- Hematologic Disorder
- Immunologic Disorder
- Positive Antinuclear Antibody
The ACR published a statement on the Methodology of Testing for Antinuclear Antibodies which states:37
- The ACR supports the immunofluorescence antinuclear antibody (ANA) test using Human Epithelial type 2 (HEp-2) substrate, as the gold standard for ANA testing.
- Hospital and commercial laboratories using alternative bead-based multiplex platforms or other solid phase assays for detecting ANAs must provide data to ordering healthcare providers on request that the alternative assay has the same or improved sensitivity compared to IF ANA.
- In-house assays for detecting ANA as well as anti-DNA, anti-Sm (anti-Smith antibodies), anti-RNP (antinuclear ribonucleoprotein), anti-Ro/SS-A (anti-Ro/Sjögren Syndrome-A), anti La/SS-B (anti-La/Sjögren Syndrome-B), etc., should be standardized according to national (e.g., CDC) and/or international (e.g., WHO, IUIS) standards.
- Laboratories should specify the methods utilized for detecting ANAs when reporting their results.
The above positions were reaffirmed in 2019.79
The ACR, together with “Choosing Wisely” also developed a list of five tests, treatments or services that are commonly used in rheumatology practice, but their value should be questioned. The ANA testing was the first on the final top five items list with level of evidence Grade 1C. In their review, the Task Force considered recommendations currently published by American College of Pathologists (ACP), ACR, and Italian Society of Laboratory Medicine (ISLM). They have issued the following recommendation: “Do not test antinuclear antibody (ANA) subserologies without a positive ANA and clinical suspicion of immune-mediated disease.”80 For their list of five things to question for pediatric rheumatology, two points pertain to ANA testing.81 “Do not order autoantibody panels unless positive ANAs and evidence of rheumatic disease. There is no evidence that autoantibody testing (including ANA and autoantibody panels) enhances the diagnosis of children with musculoskeletal pain in the absence of evidence of rheumatic disease as determined by a careful history and physical examination.” The latter recommendation also stated, “Do not repeat a confirmed positive ANA in patients with established JIA [juvenile idiopathic arthritis] or SLE.”81 These guidelines were reviewed and reaffirmed in 2021.
Rheumatoid Arthritis
In 2021, the ACR released an updated guideline on the management of rheumatoid arthritis, including new recommendations for high-risk groups. Pertaining to disease management and the risk of hepatotoxicity associated with methotrexate therapy, the ACR notes that “the use of methotrexate should be restricted to patients with normal liver enzymes and liver function tests without evidence of liver disease or liver fibrosis.” No multi-biomarker tests or disease activity tests (such as Vectra DA or PrismRA) were mentioned in the guideline for diagnostic or disease management indications.82
European League Against Rheumatism/American College of Rheumatology (EULAR/ACR)
Systemic Lupus Erythematosus (SLE)
The EULAR/ACR published a joint guideline to develop new classification criteria for systemic lupus erythematosus (SLE). In it, they stated that antinuclear antibodies (ANA) “at a titer of ≥1:80 on HEp-2 cells or an equivalent positive test” was to be an “entry criterion”: if absent, the condition is not SLE; if present, apply additive criteria such as leukopenia or oral ulcers. Antiphospholipid antibodies, complement proteins, and SLE-specific antibodies (anti-dsDNA antibodies, Anti-Smith antibodies) are all included as additive criteria for SLE diagnosis.83
Rheumatoid Arthritis
In 2022, an international task force was formed to address the safety and efficacy of disease-modifying antirheumatic drugs (DMARDs) and glucocorticoids (GCs) in the treatment of Rheumatoid Arthritis. The guideline focuses on treatment concerns. Regarding “biomarkers” they caution that certain biomarkers – i.e., acute phase reactants (APRs) such as CRP and other biomarkers comprising APRs “may respond independently of clinical improvement when antibodies to the IL-6 receptors, JAK inhibitors and even TNF-inhibitors are used.” The guideline does not mention multi-biomarker and disease activity tests such as Vectra DA or PrismRA.84
Systemic Lupus International Collaborating Clinics (SLICC)
The 2012 SLICC Classification Criteria for SLE splits the 17 criteria into two divisions—either clinical or immunologic. An individual scoring at least a four, including at least one clinical criterion and one immunologic criterion, is classified as having SLE. The criteria are cumulative and do not need to be concurrently expressed or present.85 Mosca, et al. (2019) also analyzed the accuracy and validity of the SLICC classification criteria, using a cohort of 616 patients, reporting an accuracy of 83.1%, sensitivity of 83.5%, and specificity of 82.4%. The criteria include the following:85
- Clinical Criteria
- Acute cutaneous lupus, such as lupus malar rash or subacute cutaneous lupus
- Chronic cutaneous lupus, such as classic discoid rash or discoid lupus/lichen planus overlap
- Nonscarring alopecia
- Oral or nasal ulcers
- Joint disease
- Serositis
- Renal criteria, such as urine protein-to-creatinine ratio representing 500 mg protein/24 hours or red blood cell casts
- Neurologic criteria, such seizures, psychosis, myelitis, and so on
- Hemolytic anemia
- Leukopenia or lymphopenia
- Thrombocytopenia
- Immunologic Criteria
- ANA
- Anti-dsDNA
- Anti-Sm
- Antiphospholipid antibodies
- Low complement (Low C3, Low C4, or Low CH50)
- Direct Coombs test in the absence of hemolytic anemia
British Columbia Rheumatoid Arthritis
The BC Rheumatoid Arthritis guideline includes a table of factors used in the diagnosis of rheumatoid arthritis. The C-Reactive Protein (CRP) or Erythrocyte Sedimentation Rate (ESR) test is noted as the “preferred test,” CRP/ESR indicates only inflammatory process but the guideline notes “low specificity.” For RF, “RF has low sensitivity and specificity for RA. Seropositive RA has a worse prognosis than seronegative RA.” Regarding anti-CCP, they write, “Anti-cyclic citrullinated protein antibodies (Anti-CCP) may have some value.”
For disease activity monitoring, “CRP is more sensitive to short term fluctuations” and “ESR elevated in many but not all with active inflammations.” Concerning monitoring, Rheumatoid Factor Latex Test (RF), “RF has low sensitivity and specificity for RA. Seropositive RA has a worse prognosis than seronegative RA.”86
National Institute for Clinical Excellence (NICE)
In a section on referral, diagnosis and investigations, NICE recommends:
- “Refer for specialist opinion anyone with suspected persistent synovitis of undetermined cause. Refer urgently even if blood tests show a normal acute-phase response or negative rheumatoid factor and if:
- The small joints of the hands or feet are affected
- More than one joint is affected, or
- There has been a delay of three months or longer between symptom onset and seeking medical advice.
[Based on high and moderate quality observational studies of early prognosis and identification or diagnosis].”
- “Offer to test for rheumatoid factor in people with suspected rheumatoid arthritis who have synovitis. [Based on high and moderate quality early identification observational studies]
- Consider measuring anticyclic citrullinated peptide antibodies in people with suspected rheumatoid arthritis if:
- They are negative for rheumatoid factor, and
- Combination therapy is being considered (see section on disease modifying antirheumatic drugs).”87
The Royal Australian College of General Practitioners (RACGP)
The RACGP provides a recommendation on diagnosing those with suspected rheumatoid arthritis: “RECOMMENDATION 4 – DIAGNOSTIC INVESTIGATIONS (Grade A)
For patients presenting with painful and swollen joints, GPs should support clinical examination with appropriate tests to exclude other forms of arthritis and other differential diagnoses, and to predict patients likely to progress to erosive disease. Base investigations should include:
- erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP)
- rheumatoid factor (RhF) and anti-cyclic citrullinated peptide (anti-CCP) antibody levels.”88
References
- AAFP. Autoimmune Rheumatic Diseases. Updated April 2019. https://www.aafp.org/dam/AAFP/images/about-us/content/Quest_SH8265_SoH_Autoimmune%20Rheumatic%20Diseases_HealthcareProviders_April_FINAL-2.pdf
- Guthridge JM, Wagner CA, James JA. The promise of precision medicine in rheumatology. Nat Med. Jul 2022;28(7):1363-1371. doi:10.1038/s41591-022-01880-6
- Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Advances in immunology. 1989;44:93-151.
- Satoh M, Chan EK, Sobel ES, et al. Clinical implication of autoantibodies in patients with systemic rheumatic diseases. Expert review of clinical immunology. Sep 2014;3(5):721-38. doi:10.1586/1744666x.3.5.721
- Tebo AE. Recent Approaches To Optimize Laboratory Assessment of Antinuclear Antibodies. Clinical and vaccine immunology : CVI. Dec 2017;24(12)doi:10.1128/cvi.00270-17
- Bloch D. Antibodies to double-stranded (ds)DNA, Sm, and U1 RNP. Updated May 7, 2024. https://www.uptodate.com/contents/antibodies-to-double-stranded-ds-dna-sm-and-u1-rnp
- Global Autoimmune Institute. The Global Landscape of Autoimmune Disease. Updated February 20, 2024. https://www.autoimmuneinstitute.org/articles/the-global-landscape-of-autoimmune-disease/
- Simon TA, Kawabata H, Ray N, Baheti A, Suissa S, Esdaile JM. Prevalence of Co-existing Autoimmune Disease in Rheumatoid Arthritis: A Cross-Sectional Study. Advances in therapy. Nov 2017;34(11):2481-2490. doi:10.1007/s12325-017-0627-3
- CDC. People with Lupus. Updated May 15, 2024. https://www.cdc.gov/lupus/data-research/index.html
- Rees F, Doherty M, Grainge MJ, Lanyon P, Zhang W. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology (Oxford). Nov 1 2017;56(11):1945-1961. doi:10.1093/rheumatology/kex260
- Zucchi D, Elefante E, Calabresi E, Signorini V, Bortoluzzi A, Tani C. One year in review 2019: systemic lupus erythematosus. Clin Exp Rheumatol. Sep-Oct 2019;37(5):715-722.
- Durcan L, O'Dwyer T, Petri M. Management strategies and future directions for systemic lupus erythematosus in adults. Lancet. Jun 8 2019;393(10188):2332-2343. doi:10.1016/s0140-6736(19)30237-5
- Finzel S, Schaffer S, Rizzi M, Voll RE. [Pathogenesis of systemic lupus erythematosus]. Z Rheumatol. Nov 2018;77(9):789-798. Pathogenese des systemischen Lupus erythematodes. doi:10.1007/s00393-018-0541-3
- Fava A, Petri M. Systemic lupus erythematosus: Diagnosis and clinical management. J Autoimmun. Jan 2019;96:1-13. doi:10.1016/j.jaut.2018.11.001
- Thong B, Olsen NJ. Systemic lupus erythematosus diagnosis and management. Rheumatology (Oxford). Apr 1 2017;56(suppl_1):i3-i13. doi:10.1093/rheumatology/kew401
- Bhana S. Antinuclear Antibodies (ANA). Updated February 2023. https://www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Antinuclear-Antibodies-ANA
- Johnson KJ, Sanchez HN, Schoenbrunner N. Defining response to TNF-inhibitors in rheumatoid arthritis: the negative impact of anti-TNF cycling and the need for a personalized medicine approach to identify primary non-responders. Clin Rheumatol. Nov 2019;38(11):2967-2976. doi:10.1007/s10067-019-04684-1
- Luan H, Gu W, Li H, et al. Serum metabolomic and lipidomic profiling identifies diagnostic biomarkers for seropositive and seronegative rheumatoid arthritis patients. J Transl Med. Dec 7 2021;19(1):500. doi:10.1186/s12967-021-03169-7
- Pappas DA, St John G, Etzel CJ, et al. Comparative effectiveness of first-line tumour necrosis factor inhibitor versus non-tumour necrosis factor inhibitor biologics and targeted synthetic agents in patients with rheumatoid arthritis: results from a large US registry study. Ann Rheum Dis. Jan 2021;80(1):96-102. doi:10.1136/annrheumdis-2020-217209
- Scherer HU, Häupl T, Burmester GR. The etiology of rheumatoid arthritis. J Autoimmun. Jun 2020;110:102400. doi:10.1016/j.jaut.2019.102400
- Cohen S, Wells AF, Curtis JR, et al. A Molecular Signature Response Classifier to Predict Inadequate Response to Tumor Necrosis Factor-α Inhibitors: The NETWORK-004 Prospective Observational Study. Rheumatol Ther. Sep 2021;8(3):1159-1176. doi:10.1007/s40744-021-00330-y
- Baker JF. Diagnosis and differential diagnosis of rheumatoid arthritis. Updated March 21, 2024. https://www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-rheumatoid-arthritis
- Taylor P, Maini R. Investigational biologic markers in the diagnosis and assessment of rheumatoid arthritis. Updated April 22, 2024. https://www.uptodate.com/contents/investigational-biologic-markers-in-the-diagnosis-and-assessment-of-rheumatoid-arthritis
- McArdle A, Flatley B, Pennington SR, FitzGerald O. Early biomarkers of joint damage in rheumatoid and psoriatic arthritis. Arthritis Res Ther. Jun 1 2015;17(1):141. doi:10.1186/s13075-015-0652-z
- Curtis JR, van der Helm-van Mil AH, Knevel R, et al. Validation of a novel multibiomarker test to assess rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken). Dec 2012;64(12):1794-803. doi:10.1002/acr.21767
- Centola M, Cavet G, Shen Y, et al. Development of a Multi-Biomarker Disease Activity Test for Rheumatoid Arthritis. PLoS One. 2013;8(4)doi:10.1371/journal.pone.0060635
- Shmerling R. Rheumatoid factor: Biology and utility of measurement. Updated January 27, 2025. https://www.uptodate.com/contents/rheumatoid-factor-biology-and-utility-of-measurement
- Shapiro SC. Biomarkers in Rheumatoid Arthritis. Cureus. May 16 2021;13(5):e15063. doi:10.7759/cureus.15063
- Castro CTd, Queiroz MJd, Albuquerque FC, et al. Real-world effectiveness of biological therapy in patients with rheumatoid arthritis: Systematic review and meta-analysis. Systematic Review. Frontiers in Pharmacology. 2022-August-11 2022;13doi:10.3389/fphar.2022.927179
- Bergman MJ, Kivitz AJ, Pappas DA, et al. Clinical Utility and Cost Savings in Predicting Inadequate Response to Anti-TNF Therapies in Rheumatoid Arthritis. Rheumatol Ther. Dec 2020;7(4):775-792. doi:10.1007/s40744-020-00226-3
- Curtis JR, Schabert VF, Harrison DJ, et al. Estimating effectiveness and cost of biologics for rheumatoid arthritis: application of a validated algorithm to commercial insurance claims. Clin Ther. Jul 1 2014;36(7):996-1004. doi:10.1016/j.clinthera.2014.05.062
- Suurmond J, Diamond B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J Clin Invest. 2015;125(6):2194-202. doi:10.1172/jci78084
- Aggarwal A. Role of autoantibody testing. Best practice & research Clinical rheumatology. Dec 2014;28(6):907-20. doi:10.1016/j.berh.2015.04.010
- Damoiseaux J, Andrade LE, Fritzler MJ, Shoenfeld Y. Autoantibodies 2015: From diagnostic biomarkers toward prediction, prognosis and prevention. Autoimmunity reviews. Jun 2015;14(6):555-63. doi:10.1016/j.autrev.2015.01.017
- Selmi C, Ceribelli A, Generali E, et al. Serum antinuclear and extractable nuclear antigen antibody prevalence and associated morbidity and mortality in the general population over 15 years. Autoimmunity reviews. Feb 2016;15(2):162-6. doi:10.1016/j.autrev.2015.10.007
- Hargraves MM, Richmond H, Morton R. Presentation of two bone marrow elements; the tart cell and the L.E. cell. Proceedings of the staff meetings Mayo Clinic. Jan 21 1948;23(2):25-8.
- ACR. Position Statement on Methodology of Testing for Antinuclear Antibodies. American College Of Rheumatology; 2015. https://primexlab.com/wp-content/uploads/2016/10/Methodology-of-Testing-Antinuclear-Antibodies-Position-Statement.pdf
- Chan EK, Damoiseaux J, de Melo Cruvinel W, et al. Report on the second International Consensus on ANA Pattern (ICAP) workshop in Dresden 2015. Lupus. Jul 2016;25(8):797-804. doi:10.1177/0961203316640920
- Bloch D. Measurement and clinical significance of antinuclear antibodies. Updated April 4, 2024. https://www.uptodate.com/contents/measurement-and-clinical-significance-of-antinuclear-antibodies
- Yoo IY, Oh JW, Cha HS, Koh EM, Kang ES. Performance of an Automated Fluorescence Antinuclear Antibody Image Analyzer. Annals of laboratory medicine. May 2017;37(3):240-247. doi:10.3343/alm.2017.37.3.240
- Wallace DJ, Gladman D. Systemic lupus erythematosus in adults: Clinical manifestations and diagnosis. Updated February 12, 2025. https://www.uptodate.com/contents/systemic-lupus-erythematosus-in-adults-clinical-manifestations-and-diagnosis
- Tonutti E, Bizzaro N, Morozzi G, et al. The ANA-reflex test as a model for improving clinical appropriateness in autoimmune diagnostics. Auto Immun Highlights. 2016;7(1)doi:10.1007/s13317-016-0080-3
- AVISE. AVISE Testing Exclusively from Exagen Inc. https://avisetest.com/provider/
- Exagen. AVISE Lupus. https://exagen.com/tests/lupus/
- van der Helm-van Mil AHM, Knevel R, Cavet G, Huizinga TWJ, Haney DJ. An evaluation of molecular and clinical remission in rheumatoid arthritis by assessing radiographic progression. Rheumatology (Oxford). 2013:839-46. vol. 5.
- Progenotes Diagnostics Inc. aiSLE DX. https://www.progentec.com/aisle-dx
- KSL Diagnostics-Beutner. Seronegative Rheumatoid Arthritis Profile. https://www.beutnerlabs.com/rheumatoid-arthritis-ra-laboratory-testing
- Immco Diagnostics. Early Sjögren’s Syndrome Profile. 2017;
- van der Pol P, Bakker-Jonges LE, Kuijpers J, Schreurs MWJ. Analytical and clinical comparison of two fully automated immunoassay systems for the detection of autoantibodies to extractable nuclear antigens. Clinica chimica acta; international journal of clinical chemistry. Jan 2018;476:154-159. doi:10.1016/j.cca.2017.11.014
- Tipu HN, Bashir MM. Determination of Specificity and Pattern of Antinuclear Antibodies (ANA) in Systemic Rheumatic Disease Patients Positive for ANA Testing. J Coll Physicians Surg Pak. Jan 2018;28(1):40-43.
- Kim J, Lee W, Kim GT, et al. Diagnostic utility of automated indirect immunofluorescence compared to manual indirect immunofluorescence for anti-nuclear antibodies in patients with systemic rheumatic diseases: A systematic review and meta-analysis. Seminars in arthritis and rheumatism. Feb 2019;48(4):728-735. doi:10.1016/j.semarthrit.2018.03.015
- Dervieux T, Conklin J, Ligayon JA, et al. Validation of a multi-analyte panel with cell-bound complement activation products for systemic lupus erythematosus. J Immunol Methods. Jul 2017;446:54-59. doi:10.1016/j.jim.2017.04.001
- Putterman C, Furie R, Ramsey-Goldman R, et al. Cell-bound complement activation products in systemic lupus erythematosus: comparison with anti-double-stranded DNA and standard complement measurements. Lupus Sci Med. 2014;1(1):e000056. doi:10.1136/lupus-2014-000056
- Ramsey-Goldman R, Alexander RV, Massarotti EM, et al. Complement Activation in Patients With Probable Systemic Lupus Erythematosus and Ability to Predict Progression to American College of Rheumatology-Classified Systemic Lupus Erythematosus. Arthritis Rheumatol. Jan 2020;72(1):78-88. doi:10.1002/art.41093
- Oglesby A, Korves C, Laliberté F, et al. Impact of early versus late systemic lupus erythematosus diagnosis on clinical and economic outcomes. Appl Health Econ Health Policy. Apr 2014;12(2):179-90. doi:10.1007/s40258-014-0085-x
- Yeo AL, Le S, Ong J, et al. Utility of repeated antinuclear antibody tests: a retrospective database study. The Lancet Rheumatology. 2020/07/01/ 2020;2(7):e412-e417. doi:10.1016/S2665-9913(20)30084-9
- Deng X, Peters B, Ettore MW, et al. Utility of Antinuclear Antibody Screening by Various Methods in a Clinical Laboratory Patient Cohort. J Appl Lab Med. Jul 1 2016;1(1):36-46. doi:10.1373/jalm.2016.020172
- Alsaed OS, Alamlih LI, Al-Radideh O, Chandra P, Alemadi S, Al-Allaf AW. Clinical utility of ANA-ELISA vs ANA-immunofluorescence in connective tissue diseases. Sci Rep. Apr 15 2021;11(1):8229. doi:10.1038/s41598-021-87366-w
- Wallace DJ, Alexander RV, O'Malley T, et al. Randomised prospective trial to assess the clinical utility of multianalyte assay panel with complement activation products for the diagnosis of SLE. Lupus science & medicine. 2019;6(1):e000349. doi:10.1136/lupus-2019-000349
- Mossell J, Goldman JA, Barken D, Alexander RV. The Avise Lupus Test and Cell-bound Complement Activation Products Aid the Diagnosis of Systemic Lupus Erythematosus. Open Rheumatol J. 2016;10:71-80. doi:10.2174/1874312901610010071
- Liang E, Taylor M, McMahon M. Utility of the AVISE Connective Tissue Disease test in predicting lupus diagnosis and progression. Lupus Science & Medicine. 2020;7(1):e000345. doi:10.1136/lupus-2019-000345
- Alexander RV, Rey DS, Conklin J, et al. A multianalyte assay panel with cell-bound complement activation products demonstrates clinical utility in systemic lupus erythematosus. Lupus Sci Med. Jul 2021;8(1)doi:10.1136/lupus-2021-000528
- Clarke AE, Weinstein A, Piscitello A, et al. Evaluation of the Economic Benefit of Earlier Systemic Lupus Erythematosus (SLE) Diagnosis Using a Multivariate Assay Panel (MAP). ACR Open Rheumatology. 2020;n/a(n/a)doi:10.1002/acr2.11177
- Mellors T, Withers JB, Ameli A, et al. Clinical Validation of a Blood-Based Predictive Test for Stratification of Response to Tumor Necrosis Factor Inhibitor Therapies in Rheumatoid Arthritis Patients. Network and Systems Medicine. July 14, 2020 2020;3(1):91-104. doi:10.1089/nsm.2020.0007
- Hirata S, Dirven L, Shen Y, et al. A multi-biomarker score measures rheumatoid arthritis disease activity in the BeSt study. Rheumatology (Oxford). Jul 2013;52(7):1202-7. doi:10.1093/rheumatology/kes362
- Bakker MF, Cavet G, Jacobs JW, et al. Performance of a multi-biomarker score measuring rheumatoid arthritis disease activity in the CAMERA tight control study. Ann Rheum Dis. Oct 2012;71(10):1692-7. doi:10.1136/annrheumdis-2011-200963
- Li W, Sasso EH, Emerling D, Cavet G, Ford K. Impact of a multi-biomarker disease activity test on rheumatoid arthritis treatment decisions and therapy use. Current medical research and opinion. Jan 2013;29(1):85-92. doi:10.1185/03007995.2012.753042
- Li W, Sasso EH, van der Helm-van Mil AH, Huizinga TW. Relationship of multi-biomarker disease activity score and other risk factors with radiographic progression in an observational study of patients with rheumatoid arthritis. Rheumatology (Oxford). Feb 2016;55(2):357-66. doi:10.1093/rheumatology/kev341
- Curtis JR, Greenberg JD, Harrold LR, Kremer JM, Palmer JL. Influence of obesity, age, and comorbidities on the multi-biomarker disease activity test in rheumatoid arthritis. Seminars in arthritis and rheumatism. Feb 2018;47(4):472-477. doi:10.1016/j.semarthrit.2017.07.010
- Curtis JR, Flake DD, Weinblatt ME, et al. Adjustment of the multi-biomarker disease activity score to account for age, sex and adiposity in patients with rheumatoid arthritis. Rheumatology (Oxford). May 1 2019;58(5):874-883. doi:10.1093/rheumatology/key367
- Brahe CH, Ostergaard M, Johansen JS, et al. Predictive value of a multi-biomarker disease activity score for clinical remission and radiographic progression in patients with early rheumatoid arthritis: a post-hoc study of the OPERA trial. Scand J Rheumatol. Jan 2019;48(1):9-16. doi:10.1080/03009742.2018.1464206
- Ma MHY, Defranoux N, Li W, et al. A multi-biomarker disease activity score can predict sustained remission in rheumatoid arthritis. Arthritis Research & Therapy. 2020/06/24 2020;22(1):158. doi:10.1186/s13075-020-02240-w
- Curtis JR, Weinblatt ME, Shadick NA, et al. Validation of the adjusted multi-biomarker disease activity score as a prognostic test for radiographic progression in rheumatoid arthritis: a combined analysis of multiple studies. Arthritis Research & Therapy. 2021/01/04 2021;23(1):1. doi:10.1186/s13075-020-02389-4
- Fleischmann R, Liu J, Zhu J, Segurado OG, Furst DE. Discrepancy Between Multibiomarker Disease Activity and Clinical Disease Activity Scores in Patients With Persistently Active Rheumatoid Arthritis. Arthritis Care Res (Hoboken). Sep 2022;74(9):1477-1483. doi:10.1002/acr.24583
- Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. Sep 1997;40(9):1725. doi:10.1002/art.1780400928
- CDC. Lupus Basics. Updated May 15, 2024. https://www.cdc.gov/lupus/about/index.html
- Mosca M, Costenbader KH, Johnson SR, et al. Brief Report: How Do Patients With Newly Diagnosed Systemic Lupus Erythematosus Present? A Multicenter Cohort of Early Systemic Lupus Erythematosus to Inform the Development of New Classification Criteria. Arthritis Rheumatol. Jan 2019;71(1):91-98. doi:10.1002/art.40674
- ACR. 1997 Update of the 1982 American College of Rheumatology Revised Criteria for Classification of Systemic Lupus Erythematosus. https://www.rheumatology.org/Portals/0/Files/1997%20Update%20of%201982%20Revised.pdf
- ACR. Policy & Position Statements. https://rheumatology.org/policy-position-statements
- Yazdany J, Schmajuk G, Robbins M, et al. Choosing wisely: The American College of Rheumatology's top 5 list of things physicians and patients should question. Arthritis Care & Research. 2013;65(3):329-339. doi:10.1002/acr.21930
- Rouster-Stevens KA, Ardoin SP, Cooper AM, et al. Choosing Wisely: the American College of Rheumatology's Top 5 for pediatric rheumatology. Arthritis Care Res (Hoboken). May 2014;66(5):649-57. doi:10.1002/acr.22238
- Fraenkel L, Bathon JM, England BR, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken). Jul 2021;73(7):924-939. doi:10.1002/acr.24596
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Ann Rheum Dis. Sep 2019;78(9):1151-1159. doi:10.1136/annrheumdis-2018-214819
- Josef SS, Robert BML, Sytske Anne B, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Annals of the Rheumatic Diseases. 2023;82(1):3. doi:10.1136/ard-2022-223356
- Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. Aug 2012;64(8):2677-86. doi:10.1002/art.34473
- British Columbia Rheumatoid Arthritis. Diagnosis, Management and Monitoring Guideline. https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/bc-guidelines/rheumatoid-arthritis
- Deighton C, O'Mahony R, Tosh J, Turner C, Rudolf M. Management of rheumatoid arthritis: summary of NICE guidance. Bmj. Mar 16 2009;338:b702. doi:10.1136/bmj.b702
- RACGP. Recommendations for the diagnosis and management of early rheumatoid arthritis. https://www.racgp.org.au/FSDEDEV/media/documents/Clinical%20Resources/Guidelines/Joint%20replacement/Rheumatoid-arthritis-recommendations.pdf
Coding Section
| Code | Number |
Description |
| CPT | 81490 |
Autoimmune (rheumatoid arthritis), analysis of 12 biomarkers using immunoassays, utilizing serum, prognostic algorithm reported as a disease activity score |
| 81599 |
Unlisted multianalyte assay with algorithmic analysis |
|
| 86038 |
Antinuclear antibodies (ANA) |
|
| 86039 |
Antinuclear antibodies (ANA); titer |
|
| 86200 | Cyclic citrullinated peptide (CCP), antibody | |
| 86225 |
Deoxyribonucleic acid (DNA) antibody; native or double stranded |
|
| 86235 |
Extractable nuclear antigen, antibody to, any method (e.g., nRNP, SS-A, SS-B, Sm, RNP, Sc170, J01), each antibody |
|
| 86430 | Rheumatoid factor; qualitative | |
| 86431 | Rheumatoid factor; quantitative | |
| 0039U |
Deoxyribonucleic acid (DNA) antibody, double stranded, high avidity |
|
| 0062U |
Autoimmune (systemic lupus erythematosus), IgG and IgM analysis of 80 biomarkers, utilizing serum, algorithm reported with a risk score |
|
| 0312U |
Autoimmune diseases (e.g., systemic lupus erythematosus [SLE]), analysis of 8 IgG autoantibodies and 2 cell-bound complement activation products using enzyme-linked immunosorbent immunoassay (ELISA), flow cytometry and indirect immunofluorescence, serum, or plasma and whole blood, individual components reported along with an algorithmic SLE-likelihood assessment |
|
| 0446U (effective 04/01/2024) | Autoimmune diseases (systemic lupus erythematosus [SLE]), analysis of 10 cytokine soluble mediator biomarkers by immunoassay, plasma, individual components reported with an algorithmic risk score for current disease activity | |
| 0447U (effective 04/01/2024) | Autoimmune diseases (systemic lupus erythematosus [SLE]), analysis of 11 cytokine soluble mediator biomarkers by immunoassay, plasma, individual components reported with an algorithmic prognostic risk score for developing a clinical flare | |
| 0521U (effective 01/01/2025) | Therapeutic drug monitoring, 200 or more drugs or substances, LC-MS/MS, plasma, qualitative and quantitative therapeutic minimally effective range of prescribed and non-prescribed medications. (effective 01/01/2025) |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2016 Forward
| 04/03/2025 | Annual review, editing allowed frequency of testing in coverage criteria 1,2,3. Adding new coverage criteria #10 "for testing not discussed above is considered not medically necessary". Adding ailse DX disease activity index and Early Sjogren's Syndrome Profile to new coverage criteria #13. Also updating description, table of terminology, guidelines/recommendations, and coding descriptions. |
| 11/13/2024 | Coding section updated. Code 0521U was added to coding section. This code will be effective 01/01/2025. |
| 06/25/2024 | Interim review to add PLA code 0456U to coding section. |
| 05/01/2024 | Annual review, policy updated to add coverage criteria #3 for individuals with painful and swollen joints and clinical suspicion of rheumatoid arthritis testing for RF and/or anti-CCP is medically necessary. Adding CPT 86200, 86430 and 86431. Also, updating rationale and references. |
| 03/26/2024 | Updating Coding section. Adding CPT codes 0446U and 0447U effective 04/01/2024. No other changes made. |
| 04/06/2023 | Annual review with major revision. Policy title changing from ANA/ENA testing to Biomarker Testing for Autoimmune Rheumatic Disease, lifetime testing and repeat testing addressed information regarding biomarker panel testing added. Also updating description rationale, references and coding. |
| 04/01/2022 | Annual review, no change to policy intent. Updating rationale and references and adding table of terminology. |
| 04/01/2021 | Annual review, no change to policy guidelines. Updating rationale and references. |
| 01/12/2021 | Interim review, adding policy statement regarding cell-bound, complement activation products. Also updating description, rationale and references. |
| 04/08/2020 | Annual review, no change to policy intent. Adding additional antibiodies for ENA testing, testing for Sjorgren's syndrome and verbiage regarding testing during wellness visits. |
| 04/02/2019 | Annual review, adding the following policy statement: Testing of specific antibodies when ANA test is negative or low positive MEETS COVERAGE CRITERIA only in the following situations: Testing for Anti-Jo-1 in unique clinical subset of myositis, Testing for Anti-SSA in the setting of lupus or Sjörgren’s syndrome. Updated coding secection |
| 04/17/2018 | Annual review, no change to policy intent. |
| 04/26/2017 | Interim review to align with Avalon quarterly schedule. Updated category to Laboratory. |
| 03/20/2017 | Interim review, updating policy for clarity. |
| 03/07/2017 | Updated coding section. |
| 01/19/2017 | Corrected typo in the Policy section. |
| 01/04/2017 | Annual review, no change to policy intent. |
| 01/07/2016 | NEW POLICY |