Influenza Vaccine - CAM 015
Description:
The Influenza virus vaccine imparts immunity against the influenza virus by stimulating production of antibodies that are specific to the disease. Patients who receive the
vaccine will be immune only to those strains of the virus from which the vaccine was prepared. Influenza viruses are recognized by the surface antigens they carry, and two
such antigens, hemagglutinin (H) and neuraminidase (N) have been identified and are used to classify the various viruses. Subtypes of these strains (H1, H2, H3, N1, N2) are
associated with influenza A virus and have been recognized to cause disease in humans. Immunity to these surface antigens increases resistance to infection and decreases the severity of the disease if infection occurs. Eventually, antigenic variation can occur, and immunity to one strain may no longer impart immunity to distantly related subtypes of the virus. Influenza B viruses also exhibit antigenic variation, and new variants of both types of viruses continue to cause widespread epidemics of respiratory disease. The below list provides available influenza vaccine at the development of this publication.
Background
The CDC states that the best way to prevent the flu is by getting a flu vaccine each year. All individuals ≥ 6 months of age should receive the flu vaccine annually.
Influenza (the flu) is a contagious respiratory illness caused by influenza viruses. It can cause mild to severe illness, and at times can lead to death. Over a period of 30 years, between 1976 and 2006, the flu has led to 3,000 up to 49,000 deaths each year and on average 226,000 hospitalizations each year. Seasonal influenza may also lead to death from other causes, such as pneumonia, an infection of the lungs that is usually caused by bacteria or viruses. Globally, pneumonia causes more deaths than any other infectious disease. The CDC estimates that the burden of illness during the 2017 – 2018 season was high, with an estimated 48.8 million people getting sick with influenza, 22.7 million people going to a health care provider, 959,000 hospitalizations, and 79,400 deaths from influenza. The number of cases of influenza‐associated illness that occurred in the 2017 – 2018 season was the highest since the 2009 H1N1 pandemic, when an estimated 60 million people were sick with influenza. Data regarding the 2018 – 2019 influenza season is not available at the timing of this publication.
Signs and symptoms of flu
People who have the flu often feel some or all of these signs and symptoms:
- Fever* or feeling feverish/chills
- Cough
- Sore throat
- Runny or stuffy nose
- Muscle or body aches
- Headaches
- Fatigue (very tired)
- Some people may have vomiting and diarrhea, though this is more common in children than adults.
*Not everyone with flu will have a fever.
Contraindications may include:
- Severe allergic reaction (anaphylaxis) to any component of the vaccine, including egg protein, or to a previous dose of any influenza vaccine.
- Concomitant aspirin therapy in children and adolescents (FluMist only).
The following warnings and precautions should be taken into account prior to administration of the influenza vaccine:
- Guillain‐Barré Syndrome — If Guillain-Barré syndrome has occurred within 6 weeks of receipt of prior influenza vaccine, the decision to give influenza vaccine should be based on careful consideration of the potential benefits and risks.
- Altered Immunocompetence — Immunocompromised persons, including individuals receiving immunosuppressive therapy, the expected immune response may not be obtained.
- Latex Allergy — The prefilled syringe tip caps for Fluarix, Fluvirin, Fluzone, and Fluzone high dose, contain natural rubber latex which may cause allergic reactions in latex‐sensitive individuals.
- Wheezing — In clinical trials, risks of hospitalization and wheezing were increased in children younger than 2 years of age who received FluMist. Children younger than 5 years of age with recurrent wheezing and persons of any age with asthma may be at increased risk of wheezing following the administration of FluMist.
Certain patients should not receive the nasal spray flu vaccine, including:
- Children younger than two years of age and adults older than 50.
- Pregnant women (Pregnancy Category B).
- People with a medical condition that places them at higher risk for complications from influenza, including those with chronic heart or lung disease, such as asthma or reactive airways disease; people with medical conditions such as diabetes or kidney failure; or people with illnesses that weaken the immune system, or who take medications that can weaken the immune system.
- Any patient with asthma.
- Children 2 – 4 years of age who had wheezing in the past 12 months. Children or adolescents 2 through 17 years of age currently taking aspirin or aspirin-containing medication. Children or adolescents should not be given aspirin for 4 weeks after getting FluMist or FluMist Quadrivalent unless your health care provider tells you otherwise.
- Patients who have had Guillain‐Barré syndrome (GBS), a rare disorder of the nervous system, with any prior influenza vaccinations.
- People who have a severe allergy to egg proteins, chicken proteins, gentamicin, gelatin, arginine, or who are allergic to any of the nasal spray vaccine components.
Common side effects for the flu shot may include mild fever, body aches, and fatigue for a few days after the vaccine, and soreness at the injection site.
The most common side effects seen with administration of the FluMist intranasal vaccine include runny nose or nasal congestion, headache, sore throat, tiredness/weakness, muscle aches, cough, fever, and chills.
The most common side effects observed with the administration of Fluzone Intradermal vaccine include injection‐site reactions, erythema, induration, swelling, pain, and pruritus. Erythema, induration, swelling, and pruritus occurred more frequently following Fluzone Intradermal than Fluzone.
The Advisory Committee on Immunization Practices (ACIP) voted to recommend a preference for using the live attenuated influenza virus nasal spray instead of the flu shot in healthy children 2 –8 years of age when it is immediately available. This is based on a review of studies that suggests the nasal spray can provide better protection than the flu shot in this age group. If the nasal spray is not immediately available the flu shot should be given so that opportunities to vaccinate children are not missed.
Special Consideration Regarding Egg Allergy:
People with egg allergies can receive an licensed, recommended age‐appropriate influenza vaccine (IIV, RIV4, or LAIV4) that is otherwise appropriate. People who have a history of severe egg allergy (those who have had any symptom other than hives after exposure to egg) should be vaccinated in a medical setting, supervised by a health care provider who is able to recognize and manage severe allergic reactions. For those persons 18 – 49 years of age with an egg allergy of any severity, Flublok is available. It is an egg‐free vaccine option for those who could not previously receive a flu shot due to an egg allergy. It is the only licensed flu vaccine that does not use eggs in the manufacturing process.
Policy:
Standard or preservative-free injectable influenza vaccine is a MEDICALLY NECESSARY preventive service for members when influenza immunization is recommended by the Centers for Disease Control (CDC) Advisory Committee. The current CDC recommendation is that all persons aged 6 months or older receive influenza vaccine.
NOTE: Some plans exclude preventive services. Please check benefit plan descriptions for details on coverage.
Intranasally administered influenza vaccine is considered a MEDICALLY NECESSARY alternative to injectable influenza vaccine for immunocompetent healthy persons 2 to 49 years of age as recommended by the CDC's Advisory Committee on Immunization Practices (ACIP).
Rationale:
Timing of Vaccination
- In general, health care providers should begin offering vaccination soon after vaccine becomes available, and, if possible, by October.
- All children ages 6 months to 8 years who are recommended for two doses should receive their first dose as soon as possible after vaccine becomes available. These children should receive the second dose ≥ four weeks later.
Persons at Risk for Medical Complications Due to Influenza
Vaccination to prevent influenza is particularly important for persons who are at increased risk for severe complications from influenza, or at higher risk for influenza-related outpatient, emergency department or hospital visits. When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to the following persons (no hierarchy is implied by order of listing):
- All children aged 6 months through 59 months
- All persons aged ≥ 50 years
- Adults and children who have chronic pulmonary (including asthma) or cardiovascular (except isolated hypertension), renal, hepatic, neurological, hematologic or metabolic disorders (including diabetes mellitus)
- Persons who have immunosuppression (including immunosuppression caused by medications or by HIV infection)
- Women who are or will be pregnant during the influenza season
- Children and adolescents (aged 6 months – 18 years) who are receiving long-term aspirin therapy and who might be at risk for experiencing Reye’s syndrome after influenza virus infection
- Residents of nursing homes and other long-term care facilities
- American Indians/Alaska Natives
- Persons who are morbidly obese (BMI ≥ 40)
Persons Who Live With or Care for Persons at Higher Risk for Influenza-Related Complications
All persons aged ≥ 6 months should be vaccinated annually. Continued emphasis should be placed on vaccination of persons who live with or care for persons at higher risk for influenza-related complications. When vaccine supply is limited, vaccination efforts should focus on delivering vaccination to persons at higher risk for influenza-related complications listed above, as well as these persons:
- Health care personnel (HCP)
- Household contacts (including children) and caregivers of children aged ≤ 59 months (i.e., aged < 5 years) and adults aged ≥ 50 years, with particular emphasis on vaccinating contacts of children aged < 6 months
- Household contacts (including children) and caregivers of persons with medical conditions that put them at higher risk for severe complications from influenza
HCP and persons who are contacts of persons in these groups and who are not contacts of severely immunocompromised persons (those living in a protective environment) may receive any influenza vaccine that is otherwise indicated. People who care for the severely immunocompromised should receive either IIV or RIV3.
Influenza Vaccination for Pregnant Women
- Women who are or will be pregnant during influenza season should receive IIV. Live attenuated influenza vaccine (LAIV) is not recommended for use during pregnancy.
- Postpartum women can receive either LAIV or IIV.
- Pregnant and postpartum women do not need to avoid contact with persons recently vaccinated with LAIV.
Concurrent Administration of Influenza Vaccine With Other Vaccines
- Inactivated vaccines do not interfere with the immune response to other inactivated vaccines or to live vaccines.
- Inactivated or live vaccines can be administered simultaneously with LAIV.
- However, after administration of a live vaccine, at least 4 weeks should pass before another live vaccine is administered.
Concurrent Administration of Influenza Vaccine With Other Vaccines
- Inactivated vaccines do not interfere with the immune response to other inactivated vaccines or to live vaccines.
- Inactivated or live vaccines can be administered simultaneously with LAIV.
- However, after administration of a live vaccine, at least 4 weeks should pass before another live vaccine is administered.
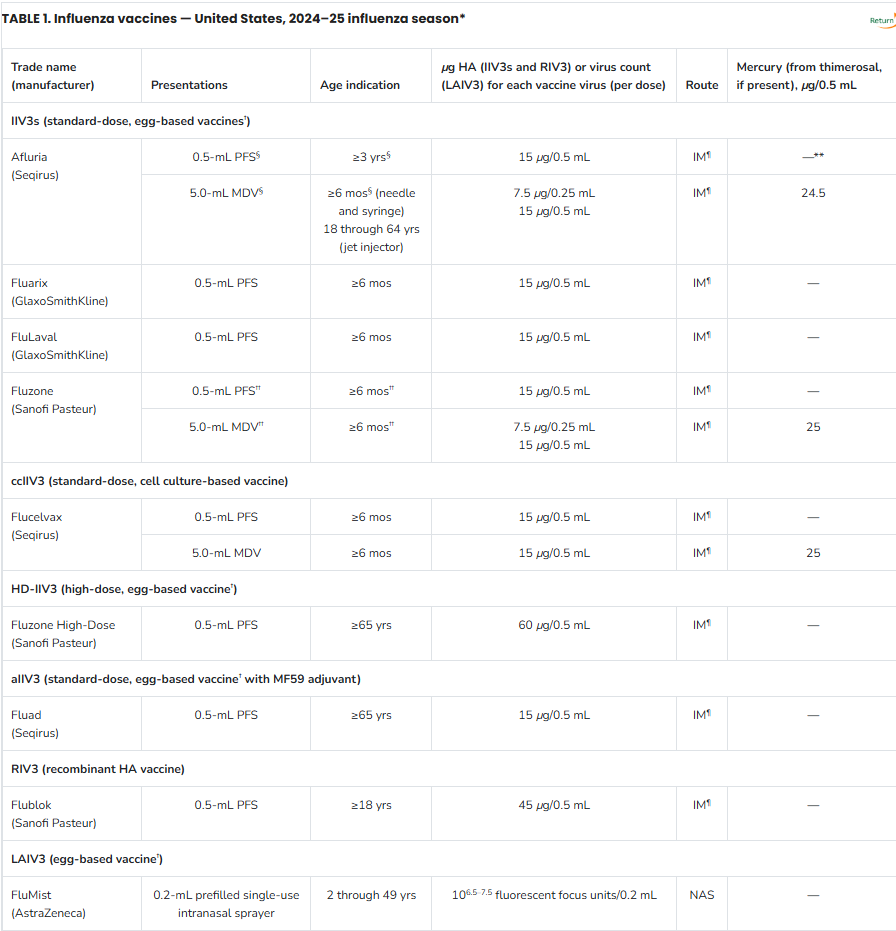
Abbreviations: ACIP = Advisory Committee on Immunization Practices; aIIV3 = adjuvanted inactivated influenza vaccine, trivalent; ccIIV3 = cell culture-based inactivated influenza vaccine, trivalent; HA = hemagglutinin; HD-IIV3 = high-dose inactivated influenza vaccine, trivalent; IIV3 = inactivated influenza vaccine, trivalent; IM = intramuscular; LAIV3 = live attenuated influenza vaccine, trivalent; MDV = multidose vial; NAS = intranasal; PFS = prefilled syringe; RIV3 = recombinant influenza vaccine, trivalent.
* Manufacturer package inserts and updated CDC and ACIP guidance should be consulted for additional information concerning, but not limited to, indications, contraindications, warnings, and precautions. Package inserts for U.S.-licensed vaccines are available at https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states. Availability and characteristics of specific products and presentations might change or differ from what is described in this table and in the text of this report.
† Although a history of severe allergic reaction (e.g., anaphylaxis) to egg is a labeled contraindication to the use of egg-based IIV3s and LAIV3, ACIP recommends that all persons aged ≥6 months with egg allergy should receive influenza vaccine and that any influenza vaccine (egg based or nonegg based) that is otherwise appropriate for the recipient’s age and health status can be used (see Persons with a History of Egg Allergy).
§ The approved dose volume for Afluria is 0.25 mL for children aged 6 through 35 months and 0.5 mL for persons aged ≥3 years. However, 0.25-mL prefilled syringes are no longer available. For children aged 6 through 35 months, a 0.25-mL dose must be obtained from a multidose vial.
¶ IM-administered influenza vaccines should be administered by needle and syringe only, except for the MDV presentation of Afluria, which can alternatively be given by the PharmaJet Stratis jet injector for persons aged 18 through 64 years only. For older children and adults, the recommended site for IM influenza vaccination is the deltoid muscle. The preferred site for infants and young children is the anterolateral aspect of the thigh. Additional specific guidance regarding site selection and needle length for IM administration is available in the General Best Practice Guidelines for Immunization available at https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html.
** Not applicable.
†† Fluzone is approved for children aged 6 through 35 months at either 0.25 mL or 0.5 mL per dose; however, 0.25-mL prefilled syringes are no longer available. If a prefilled syringe of Fluzone is used for a child in this age group, the dose volume will be 0.5 mL per dose.
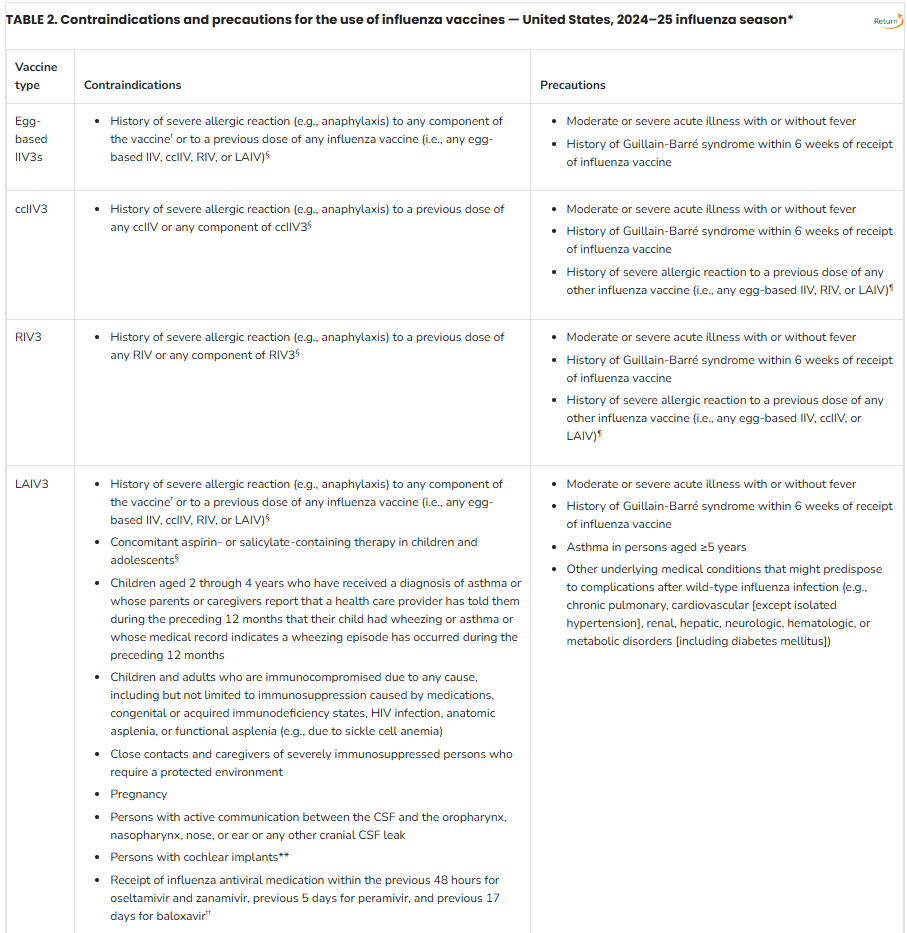
Abbreviations: ACIP = Advisory Committee on Immunization Practices; ccIIV = cell culture–based inactivated influenza vaccine (any valency); ccIIV3 = cell culture–based inactivated influenza vaccine, trivalent; CSF = cerebrospinal fluid; IIV = inactivated influenza vaccine (any valency); IIV3 = inactivated influenza vaccine, trivalent; LAIV = live attenuated influenza vaccine (any valency); LAIV3 = live attenuated influenza vaccine, trivalent; RIV = recombinant influenza vaccine (any valency); RIV3 = recombinant influenza vaccine, trivalent.
* Manufacturer package inserts and updated CDC and ACIP guidance should be consulted for additional information concerning, but not limited to, indications, contraindications, warnings, and precautions. When a contraindication is present, a vaccine should not be administered. When a precaution is present, vaccination should generally be deferred but might be indicated if the benefit of protection from the vaccine outweighs the risk for an adverse reaction (see the General Best Practice Guidelines for Immunization, available at https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/index.html). Package inserts for U.S.-licensed vaccines are available at https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states.
† Although a history of severe allergic reaction (e.g., anaphylaxis) to egg is a labeled contraindication to the use of egg-based IIV3s and LAIV3, ACIP recommends that all persons aged ≥6 months with egg allergy should receive influenza vaccine, and that any influenza vaccine (egg based or nonegg based) that is otherwise appropriate for the recipient’s age and health status can be used (see Persons with a History of Egg Allergy).
§ Labeled contraindication noted in package insert.
¶ If administered, vaccination should occur in a medical setting and should be supervised by a health care provider who can recognize and manage severe allergic reactions. Providers can consider consultation with an allergist in such cases to assist in identification of the component responsible for the allergic reaction.
** Injectable vaccines are recommended for persons with cochlear implant because of the potential for CSF leak, which might exist for a period after implantation. Providers might consider consultation with a specialist concerning risk for persistent CSF leak if an inactivated or recombinant vaccine cannot be used.
†† Use of LAIV3 in context of influenza antivirals has not been studied; however, interference with activity of LAIV3 is biologically plausible, and this possibility is noted in the package insert for LAIV3. In the absence of data supporting an adequate minimum interval between influenza antiviral use and LAIV3 administration, the intervals provided are based on the half-life of each antiviral. The interval between influenza antiviral receipt and LAIV3 for which interference might potentially occur might be further prolonged in the presence of medical conditions that delay medication clearance (e.g., renal insufficiency). Influenza antivirals might also interfere with LAIV3 if initiated within 2 weeks after vaccination. Persons who receive antivirals during the period starting with the specified time before receipt of LAIV3 through 2 weeks after receipt of LAIV3 should be revaccinated with an age-appropriate IIV3 or RIV3.
References:
- Bauchner H. Journal Watch Pediatrics and Adolescents. Flu Shots or Intranasal Flu Vaccine for Children? Reviewed Oct 2008.
- Huffman G. American Family Practice. Effectiveness of Intranasal Influenza Vaccine. January 15, 2000.
- Nichol K, et al. Effectiveness of live, attenuated intranasal influenza virus vaccine in healthy, working adults. A randomized controlled trial. JAMA July 14, 1999;282: 137-44.
- Smith NM, Bresee JS, Shay DK, et al. Advisory Committee of Immunization Practices. Prevention and Control of Influenza: Recommendations of the Advisory Committee on Immunization Practice (ACIP). MMWR Recomm Rep. 2006;55 (RR-10):1-42.
- Loeb M. Community acquired pneumonia. In: BMJ Clinical Evidence. London, UK: BMJ Publishing Group; April 2007.
- U.S. Food and Drug Administration (FDA). FDA approves nasal influenza vaccine for use in younger children. FDA News. Rockville, MD: FDA; September 19, 2007.
- U.S. Food and Drug Administration (FDA). Additional influenza vaccine approved for upcoming season. Approval increases number of available doses to record level. FDA News. Rockville, MD: FDA; September 28, 2007.
- Lewis EN, Friffin MR, Szilagy PG, et al. Childhood influenza. Number need to vaccinate to prevent 1 hospitalization or outpatient visit. Pediatrics. 2007;120(3): 467-472.
- Treanor JD. Influenza-The goal of control. N Engl J Med. 2007; 357(14):1439-1441.
- Centers of Disease Control and Prevention (CDC). Division of Media Relations. CDC's advisory committee recommends influenza vaccination for children 6 months through 18 years of age. Press Release. Atlanta, GA; CDC; February 27, 2008.
- Fiore AE, Shay DK, Broder K, et al. Centers for Disease Control and Prevention (CDC): Advisory Committee on Immunization Practices (ACIP). Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). 2008. MMWR Recomm Rep. 2008;57 (RR-7):1-60.
- U.S. Food and Drug Administration (FDA). FDA Approve 2008-2009 Flu Vaccines. FDA News. Rockville, MD: FDA ; August 5, 2008.
- http://www.cdc.gov/flu/professionals/acip/2013-summary-recommendations.htms
- http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6430a.htm
- CDC. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices [ACIP]. https://www.cdc.gov/flu/professionals/acip/. Accessed August 2021.
- Clinical Pharmacology [database online]. Tampa, FL: Gold Standard, Inc.; 2021. URL: http://www.clinicalpharmacology.com Accessed August 2021.
- Hurwitz ES, Schonberger LB, Nelson DB, Holman RC. Guillain‐Barré syndrome and the 1978‐1979 influenza vaccine. N Engl J Med 1981;304:1557‐1561.
- Centers for Disease Control and Prevention. (2021). Influenza (Flu). URL: https://www.cdc.gov/flu/index.htm Accessed August 2021
Coding Section
| Codes | Number | Description |
| CPT | 90460 | Immunization administration through 18 years of age via any route of administration, with counseling by physician or other qualified health care professional; first or only component of each vaccine or toxoid administered |
| 90461 | Immunization administration through 18 years of age via any route of administration, with counseling by physician or other qualified health care professional; each additional vaccine or toxoid component administered (List separately in addition to code for primary procedure) |
|
| 90471 | Immunization administration (includes percutaneous, intradermal, subcutaneous, or intramuscular injections); one vaccine (single or combination vaccine/toxoid) | |
| 90472 | Immunization administration (includes percutaneous, intradermal, subcutaneous, or intramuscular injections); each additional vaccine (single or combination vaccine/toxoid) (List separately in addition to code for primary procedure) | |
| 90473 | Immunization administration by intranasal or oral route; one vaccine (single or combination vaccine/toxoid) | |
| 90474 | Immunization administration by intranasal or oral route; each additional vaccine (single or combination vaccine/toxoid) (List separately in addition to code for primary procedure) | |
| 90630 | Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, for intradermal use | |
| 90653 | Influenza vaccine, inactivated (IIV), subunit, adjuvanted, for intramuscular use | |
| 90654 | Influenza virus vaccine, trivalent (IIV3), split virus, preservative-free, for intradermal use | |
| 90655 | Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, when administered to children 6 – 35 months of age, for intramuscular use | |
| 90655 (effective 1/1/2017) | Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, 0.25 mL dosage, for intramuscular use | |
| 90656 | Influenza virus vaccine, trivalent (IIV3), split virus, preservative free, 0.5-mL dosage, for intramuscular use. | |
| 90657 | Influenza virus vaccine, trivalent (IIV3), split virus, when administered to children 6-35 months of age, for intramuscular use | |
| 90657 (effective 8/1/2016) | Influenza virus vaccine, trivalent (IIV3), split virus, 0.25 mL dosage, for intramuscular use | |
| 90658 | Influenza virus vaccine, trivalent (IIV3), split virus, when administered to individuals 3 years of age and older, for intramuscular use | |
| 90658 (effective 1/1/2017) | Influenza virus vaccine, trivalent (IIV3), split virus, 0.5 mL dosage, for intramuscular use | |
| 90660 | Influenza virus vaccine, trivalent, live (LAIV3), for intranasal use | |
| 90661 | Influenza virus vaccine (ccIIV3), derived from cell cultures, subunit, preservative and antibiotic free, for intramuscular use | |
| 90661 (effective 8/1/2016) | Influenza virus vaccine, trivalent (ccIIV3), derived from cell cultures, subunit, preservation and antibiotic free, 0.5 mL dosage, for intramuscular use | |
| 90662 | Influenza virus vaccine (IIV), split virus, preservative free, enhanced immunogenicity via increased antigen content, for intramuscular use | |
| 90664 | Influenza virus vaccine, live (LAIV), pandemic formulation, for intranasal us | |
| 90666 | Influenza virus vaccine (IIV), pandemic formulation, split virus, preservative free, for intramuscular use | |
| 90667 | Influenza virus vaccine (IIV), pandemic formulation, split virus, adjuvanted, for intramuscular use | |
| 90668 | Influenza virus vaccine (IIV), pandemic formulation, split virus, for intramuscular use | |
| 90672 | Influenza virus vaccine, quadrivalent, live (LAIV4), for intranasal use | |
| 90673 | Influenza virus vaccine, trivalent (RIV3), derived from recombinant DNA (RIV3), hemagglutinin (HA) protein only, preservative and antibiotic free, for intramuscular use | |
| 90674 (effective 8/1/2016) | Influenza virus vaccine, quadrivalent (ccIIV4), derived from cell cultures, subnit, preservative and antibiotic free, 0.5 mL dosage, for intramuscular use | |
| 90682 (effective 1/01/2018) | INFLUENZA VIRUS VACCINE, QUADRIVALENT (RIV4), DERIVED FROM RECOMBINANT DNA, HEMAGGLUTININ (HA) PROTEIN ONLY, PRESERVATIVE AND ANTIBIOTIC FREE, FOR INTRAMUSCULAR USE | |
| 90685 | Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, when administered to children 6 – 35 months of age, for intramuscular use | |
| 90685 (effective 8/1/2016) | Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, 0.25 mL dosage, for intramuscular use | |
| 90686 | Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, when administered to individuals 3 years of age and older, for intramuscular use | |
| 90686 (effective 8/1/2016) | Influenza virus vaccine, quadrivalent (IIV4), split virus, preservative free, 0.5 mL dosage, for intramuscular use | |
| 90687 | Influenza virus vaccine, quadrivalent (IIV4), split virus, when administered to children 6 – 35 months of age, for intramuscular use | |
| 90687 (effective 8/1/2016) | Influenza virus vaccine, quadrivalent (IIV4), split virus, 0.25 mL dosage, for intramuscular use | |
| 90688 | Influenza virus vaccine, quadrivalent (IIV4), split virus, when administered to individuals 3 years of age and older, for intramuscular use | |
| 90688 (effective 8/1/2016) | Influenza virus vaccine, quadrivalent (IIV4), split virus, 0.5 mL dosage, for intramuscular use | |
| 90694 | INFLUENZA VIRUS VACCINE, QUADRIVALENT (AIIV4), INACTIVATED, ADJUVANTED, PRESERVATIVE FREE, 0.5 ML DOSAGE, FOR INTRAMUSCULAR USE | |
| 90756 (effective 1/1//2018) | Influenza virus vaccine, quadrivalent (ccIIV4), derived from cell cultures, subunit, antibiotic free, 0.5mL dosage, for intramuscular use | |
| HCPCS | G0008 | Administration of influenza virus vaccine |
| G0009 | Administration of pneumococcal vaccine | |
| G0010 | Administration of hepatitis B vaccine | |
| Q2035 | Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (AFLURIA) | |
| Q2036 | Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (FLULAVAL) | |
| Q2037 | Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (FLUVIRIN) | |
| Q2038 | Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (Fluzone) | |
| Q2039 | Influenza virus vaccine, split virus, when administered to individuals 3 years of age and older, for intramuscular use (not otherwise specified) |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2014 Forward
| 09/01/2025 | Annual review, no change to policy intent. |
| 01/30/2025 | Interim review. Updated table 1 and table 2. No other changes made. |
| 10/07/2024 | Updating coding verbiage for 90656. |
| 09/03/2024 | Annual review, no change to policy intent. |
| 09/07/2023 | Annual review, updating table one and two for ACIP 2023/2024 recommendations. |
| 09/01/2022 | Annual review, no change to policy intent. Updating description and references. Adding background. |
| 09/01/2021 |
Annual review, no change to policy intent. |
| 09/01/2020 |
Annual review, no change to policy intent. Updating summary of evidence, background, rationale and references. |
| 06/08/2020 |
Correcting typo in history box dated 5/28/2020. Added code 90694 to coding section. No other changes made. |
| 05/28/2020 |
Adding code 90967 to coding section. No other changes made. |
| 09/01/2019 |
Annual review, no change to policy intent. |
| 09/05/2018 |
Annual review, no change to policy intent. |
| 02/07/2018 |
Correction of date in coding section. No other changes made. |
| 08/24/2017 |
Adding Code 90756 to coding section. No change to policy intent. |
| 03/01/2017 |
Updated CPT coding. |
| 09/19/2016 |
Updated CPT coding effective date. Updated rationale tables. |
| 08/03/2016 |
Updated CPT coding in policy. |
| 08/31/2015 |
Annual review, no change to policy intent. Adding coding and updating table 1 to current ACIP recommendations. |
| 08/18/2015 |
Changed review to September, as the 2015/2016 CDC recommendations have not been released yet. |
| 08/11/2014 |
Annual review, no changes made. |