Molecular Markers in Fine Needle Aspirates of the Thyroid - CAM 279
Description
Thyroid nodules are growths or enlargements on the thyroid gland. These nodules may be caused by several different disorders such as thyroiditis or cysts. However, these nodules may also be caused by thyroid cancer, which occurs in 4-6.5% of nodules. A biopsy is often performed to assess the histological components of this nodule, usually through a fine needle aspiration (FNA). A 23 to 27-gauge (commonly 25 gauge) needle is used, with or without local anesthesia. This technique can obtain an acceptable sample in 90-97% of solid nodules.1
Molecular markers, such as genetic mutations or microRNA (miRNA) expression, may be used to help identify malignant nodules. Mutational analysis by sequencing or PCR can identify mutations, such as BRAF, RAS, RET/PTC, and PAX8/PPARG. Another tool is a gene expression classifier, which measures mRNA to determine the activity level of several genes and uses an algorithm to predict malignancy based on gene expression.2
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
- To assist in patient management decisions for individuals 18 years of age or older being evaluated for thyroid carcinoma, mutation analysis (e.g., BRAF V600, RET/PTC, RAS, PAX8/PPARG) and/or the use of a gene expression classifier in fine-needle aspirates (FNAs) of the thyroid is considered MEDICALLY NECESSARY when the FNA is cytologically characterized as any of the following:
- Bethesda-III (atypia of undetermined significance [AUS] or follicular lesion of undetermined significance [FLUS])
- Bethesda-IV (follicular neoplasm [FN] or suspicious for follicular neoplasm [SFN])
- For individuals 18 years of age or older being evaluated for thyroid carcinoma, mutation analysis (e.g., BRAF V600, RET/PTC, RAS, PAX8/PPARG) and/or the use of a gene expression classifier in FNAs of the thyroid) is considered NOT MEDICALLY NECESSARY when the FNA is cytologically characterized as any of the following:
- Bethesda-I (nondiagnostic or unsatisfactory)
- Bethesda-II (benign)
- Bethesda-V (suspicious for malignancy)
- Bethesda-VI (malignant)
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of a patient’s illness.
- For individuals under 18 years of age, mutation analysis (e.g., BRAF V600, RET/PTC, RAS, PAX8/PPARG) or the use of a gene expression classifier in FNAs of the thyroid is considered NOT MEDICALLY NECESSARY.
- MicroRNA profiling tests (e.g., ThyraMIR) in FNAs of the thyroid is considered NOT MEDICALLY NECESSARY.
- For all other situations not discussed above, mutation analysis (e.g., BRAF V600, RET/PTC, RAS, PAX8/PPARG) or the use of a gene expression classifier is considered NOT MEDICALLY NECESSARY.
NOTES:
Note: For two or more gene tests being run on the same platform, please refer to Reimbursement Policy, CAM 235.
Table of Terminology
| Term |
Definition |
| AACE |
American Association of Clinical Endocrinologists |
| AAP |
American Academy of Pediatrics |
| ACE |
American College of Endocrinology |
| ALK |
Anaplastic lymphoma kinase gene |
| ALS |
Argininosuccinate lyase gene |
| AME |
Associazione medici endocrinologi |
| ARID1A |
AT-rich interaction domain 1A gene |
| ATA |
American Thyroid Association |
| ATM |
ATM serine/threonine kinase gene |
| AUS |
Atypia of undetermined significance |
| BRAF |
B-Raf proto-oncogene gene |
| CDKN2A |
Cyclin dependent kinase inhibitor 2A gene |
| CLIA/CAP |
Clinical Laboratory Improvement Amendments/College of American Pathologists |
| CMS |
Centers for Medicare & Medicaid Services |
| CTNNB1 |
Catenin beta 1 gene |
| DNA |
Deoxyribonucleic acid |
| ERBB2 |
Erb-b2 receptor tyrosine kinase 2 gene |
| ERBB4 |
Erb-b2 receptor tyrosine kinase 4 gene |
| ESMO |
European Society for Medical Oncology |
| EU-TIRADS | European Thyroid Imaging Reporting and Data System |
| ETA |
European Thyroid Association |
| FISH |
Fluorescence in situ hybridization |
| FLUS |
Follicular lesion of undetermined significance |
| FN |
Follicular neoplasm |
| FNA |
Fine needle aspiration |
| FNAs |
Fine needle aspirates |
| GC |
Genomic classifier |
| GEC |
Gene expression classifier |
| GSC |
Genomic sequencing classifier |
| HRAS |
HRAS proto-oncogene |
| KRAS |
KRAS proto-oncogene |
| LDTs |
Laboratory-developed tests |
| MEN1 |
Menin 1 |
| MET |
Met proto-oncogene, receptor tyrosine kinase gene |
| MPTX |
Thygenext expanded mutation panel |
| mRNA |
Messenger ribonucleic acid |
| miRNA |
Micro-ribonucleic acid |
| MSI |
Microsatellite instability |
| MTC |
Medullary thyroid cancer |
| NCCN |
National comprehensive cancer network |
| NGS |
Next-generation sequencing |
| NIFTP |
Noninvasive follicular thyroid neoplasm with papillary-like nuclear features |
| NKX2-1 |
NK2 homeobox 1 |
| NPV |
Negative predictive value |
| NTRK |
Neurotrophic tyrosine receptor kinase gene |
| PAX8/PPARG |
Paired box gene 8/peroxisome proliferator-activated receptor gamma gene |
| PCR |
Polymerase chain reaction |
| PD-L1 |
Programmed death-ligand 1 |
| PIK3CA |
Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha gene |
| PTEN |
Phosphatase and tensin homolog gene |
| PPV |
Positive predictive value |
| RAS |
Rat sarcoma virus |
| RET/PTC |
Rearranged during transfection)/papillary thyroid carcinoma type 1 gene |
| RNA |
Ribonucleic acid |
| RT-PCR |
Reverse transcriptase polymerase chain reaction |
| SFN |
Suspicious for follicular neoplasm |
| SMAD4 |
SMAD family member 4 gene |
| SMO |
Smoothened, frizzled class receptor gene |
| SRC |
Proto-oncogene tyrosine-protein kinase Src gene |
| TBP |
TATA-box binding protein |
| TERT |
Telomerase reverse transcriptase gene |
| Tg | Thyroglobulin |
| TMB |
Tumor mutation burden |
| US |
Ultrasound |
| USP33 |
Ubiquitin specific peptidase 33 |
| XA |
Xpression atlas |
Rationale
Fine needle aspiration (FNA) is a traditional diagnostic approach to differentiate malignant thyroid nodules that need surgery from benign nodules that do not require surgery. It offers definitive diagnosis in most cases; however, 20–30% of nodules yield an indeterminate cytologic diagnosis in which cancer cannot be ruled out, and such nodules can exhibit malignancy risk ranging from 12% to 33%.3 This may lead to suboptimal management of these patients and can result in unnecessary resections and surgical interventions.2 FNA results are normally categorized according to the National Cancer Institute into six categories. They are, in order of severity: nondiagnostic or unsatisfactory (Bethesda-I), benign (II), atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS) (III), follicular neoplasm (FN) or suspicious for follicular neoplasm (SFN) (IV), suspicious for malignancy (V), and malignant, which includes lymphomas and carcinomas (VI).4 A benign result is reported in 60-70% of FNAs. Nondiagnostic does not refer to an absence of cancer; rather it means that the sample provided was inadequate for a conclusive result and another sample must be provided. Categories III and IV are typically referred to as “indeterminate” and typically have a malignancy risk of as high as 40%. Patients with indeterminate nodules will frequently have a surgery to treat the issue; however, up to 95% of these nodules are ultimately evaluated as benign. Further testing must be done with indeterminate cases to categorize these lesions.5,6
Molecular markers have been used to identify the true status of an indeterminate FNA. Assessing components of FNA aspirates, such as micro-RNA, mutational status of certain genes, or genomic sequencing could prove useful, especially as these components can be reliably detected during the FNA itself.7,8 Prior to the emergence of molecular markers as an indicator, a repeat FNA was often performed, ultimately leading to a surgery to remove the nodule. Molecular markers could thus reduce unnecessary surgeries as well as provide better risk stratification.5
Proprietary Testing
Commercially available panels of molecular markers utilizing FNA specimens from the thyroid include the following tests:
ThyGeNEXT and ThyraMIR
ThyGeNEXT (Interpace Diagnostics, Parsippany, NJ) is a specific oncogene, mutational panel that tests genetic alterations across ten genes associated with papillary carcinoma and follicular carcinoma. ThyGeNEXT uses a next generation sequencing (NGS) platform to identify genetic alterations across those ten genes, which are as follows: ALK, BRAF, GNAS, HRAS, KRAS, NRAS, PIK3CA, PTEN, RET, TERT. This test is primarily for category III or IV nodules. Recently, Interpace Diagnostics has developed a new molecular test, ThyraMIR. This test is based on microRNA analysis of the expression of 10 microRNAs. The manufacturer claims that this test can identify malignancy in nodules that otherwise evince no or “weak” mutation for ThyGeNEXT. According to Interpace Diagnostics, combined test performance has negative predictive value of 99%, positive predictive value of 96%, with 98% sensitivity and 98% specificity. The sensitivity and specificity for the ThyGeNEXT panel alone is 63% and 84%, respectively, for cases of indeterminant cytology.9
Lupo, et al. (2020) featured a blinded multicenter study centered around the performance of the ThyGeNEXT expanded mutation panel (MPTX) and the expanded panel with the microRNA classifier ThyraMIR by comparing them to the histopathology diagnosis by three pathologists. The expanded mutation panel included NTRK and ALS fusions that have targeted therapies as well as proto-oncogenes TERT and RET, which are indicative of aggressive disease. The study found that the performance of the expanded mutation panel (ThyGeNEXT) alone unsatisfactory due to numerous false positives, 90% of which were attributed to individual RAS mutations primarily found in benign adenomas, with a few additional errors due to TERT mutations in benign disease. It was therefore reported that the expanded mutation panel of ThyGeNEXT alone demonstrated an NPV of 81% while the PPV was even less stellar, a mere 56%.10 This contrasts with the reported high sensitivity for malignancy (95%) in negative mutation panel testing results and high (90%) specificity in nodules with Bethesda III and IV cytology, purportedly driving an increase in the NPV to 97% and the PPV to 75%, as aforementioned above. The researchers suggest that the increases in NPV and PPV from the inclusion of the microRNA classifier, therefore, possess great power and potential in ruling in and out the need for surgery for indeterminate thyroid nodule cytology, leading them to conclude that ancillary use of the three category MPTX approach can be leveraged to accurately inform the need for surgery in four out of five indeterminate nodules tested.10
The same optimism may be found in Jackson, et al. (2020), where researchers aimed to better understand the incremental use of using expanded mutation panels, along with the integration of microRNA classifier testing to provide additional and more accurate diagnostic information. Using molecular results from two consecutive cohorts of patients totaling 12993 members, who had FNAs performed on thyroid nodules resulting in Bethesda Diagnostic categories III or IV cytology results, underwent either focused mutation panel testing (n=8619) alone or expanded mutation panel testing (n=4374), the latter of which included the microRNA classifier test ThyraMIR. The study found that 89% of patients who underwent the simple focused panel testing lacked detectable oncogenic mutations and fusions as compared to the 74% in the cohort who underwent the expanded panel testing (P < 0.001). Moreover, weak drivers were more frequently identified in patients who underwent expanded (20%) compared with focused (9%) panel testing (P < 0.001), and strong drivers were likewise more frequent in patients who underwent expanded (6%) compared with focused (2%) panel testing (P < 0.001). Thus, the power of the expanded panel was not limited to detecting weak drivers, as 16% of those who underwent focused panel testing had strong drivers, while 24% of those who underwent expanded panel testing had strong drivers (P < 0.001). Inclusion of less common mutations in the expanded panel increased the detection of multiple coexisting mutations by 4%, which provides increased utility in identifying aggressive forms of thyroid cancer. However, the study concluded broadly that all oncogenic changes can contribute to neoplastic growth and progression, and therefore both strong and weak drivers should be considered clinically important. From the results of the study, the researchers contend that subsequent microRNA testing can help overcome uncertainty as to the presence of cancer, as approximately half of nodules with weak drivers had positive microRNA results consistent with a higher risk of malignancy, and 33% of those with positive microRNA results likewise had strong positive microRNA levels specific for malignancy that are prevalent in nodules with strong drivers.3
Ciarletto, et al. (2021) used ThyraMIR to study if pairwise comparisons of differentially expressed miRNAs can identify medullary thyroid carcinoma in FNA. Differential pairwise analysis was performed on ten miRNAs in 7557 specimens. Nine differential pairs were determined to have significant power to differentiate medullary thyroid carcinoma and non- medullary thyroid carcinoma samples. The test accuracy was 100%, “the assay correctly classified all MTC [medullary thyroid carcinoma] and non-MTC samples.” The authors conclude that “pairwise miRNA expression analysis of ThyraMIR results were found to accurately predict medullary thyroid carcinoma in thyroid FNA samples, including those with indeterminate FNA findings”.11
ThyroSeq v3
ThyroSeq is a test intended for assessment of thyroid nodules with undetermined cytology initially designed to target 12 cancer genes with 284 mutational hotspots. The latest version of this test, ThyroSeq v3, is based on NGS of DNA and RNA. This test detects four types of alterations; mutations, gene fusions, expression alterations, and copy number alterations. This test analyzes 112 genes, providing information on more than 12,000 mutation hotspots and more than 120 gene fusion types. First, the sample is ensured to have enough material to proceed (such as amount of thyroid follicular cells). Then, the NGS is performed and reviewed by a pathologist. Finally, the report is sent to the patient in about 2 weeks.12
Kim, et al. (2023) conducted a comparative study on the performance of Afirma GSC and ThyroSeq v3 in 372 indeterminate thyroid nodules. The study tested 152 nodes with ThyroSeq v3 and reported that ThyroSeq v3 had a sensitivity of 97%, indicating its high effectiveness accurately identifying malignant nodules. The specificity was found to be 83%, demonstrating its ability to correctly identify benign nodules.13
Afirma Series
The Afirma genomic sequencing classifier (GSC) is offered by Afirma. This test is intended to assess indeterminate nodules (Bethesda categories III and IV) and “conclusively rule out” surgery.14 This test is based on the Afirma Gene Expression Classifier (GEC), which measured the activity level of 167 genes. The GSC added several features to better classify nodules, such as classifiers for medullary thyroid cancer, parathyroid lesions, BRAF V600E mutations, and overall better discrimination of Hürthle cell neoplasms.5 Wei et al note that the GSC is an “updated” version of the GEC, available since 2011. Wei et al also published a study discussing proposed advantages of the GSC over the GEC, namely its improved specificity (as a weakness of the GEC was its inability to identify true oncocytic lesions in the “suspicious” category). The authors compared the results of indeterminate FNA specimens (Bethesda categories III and IV) that were tested by Afirma GEC or GSC. 272 tests (194 GEC, 78 GSC) were evaluated. 221 samples were classified as AUS/FLUS and 51 were classified as FN/SFN. Out of the 194 samples tested with GEC, 88 were considered benign (45.4%) whereas 52 of the 78 GSC samples were considered benign (66.7%). In the AUS/FLUS category, 47.1% of cases were considered benign by GEC whereas 71.2% were considered benign by GSC.15
A component of the Afirma GSC is the medullary thyroid cancer (MTC) component. This component includes 108 genes. Randolph, et al. (2022) performed a validation of this test using 211 samples (21 MTC cases, 190 controls), and all 211 samples were identified correctly. These sample results were confirmed by surgery (for positive cases) and pathology (for negative cases).16
Another test is the Afirma Xpression Atlas. This test is a comprehensive panel encompassing 511 genes, 761 variants, and 130 fusion pairs. Genes such as BRAF, RET, and the RAS pathway of genes are included in this panel. This test is intended to inform surgical and therapeutic decisions for high-risk patients. Afirma lists three populations for this test; those who are “suspicious” per the Afirma GSC test, Bethesda category V, and Bethesda category VI. Angell et al validated this test. The XA was compared to the results of multiple other methodologies (such as whole-transcriptome RNA-seq, targeted DNA-seq, et al) and concordance was evaluated. 943 blinded FNA samples were used to compare the DNA and RNA detection and 695 blinded FNAs were used to compare the fusion detections. At the cutoff of 5% variant frequency, 74% of allele variants detected by traditional methods were also detected by the XA, with 88% detected by the XA at a 20% variant frequency cutoff. 82% of fusions detected by the targeted RNA fusion assay were detected by the XA. From an analytical validity standpoint, intra-plate reproducibility was found to be 89%-94%, and inter-plate reproducibility was found to be 86%-91%.17 More recently, Krane, et al. (2020) compiled evidence for the analytical validity, clinical validity, and clinical utility of the Afirma series in an evidence-based assessment. Analytical validation of the Afirma XA test using 69 variant-positive FNA samples demonstrated that there was high accuracy between the detection of variants (90%) and detection of fusion (94%) across two different laboratories. Clinical validation on the Afirma XA test’s ability to detect genomic variants was measured against those of currently accepted methods of DNA and RNA sequencing using the aforementioned 943 FNA-blinded samples. However, it should be noted that 95% or more of the variants and fusions identified by Afirma XA can be identified simply through the reference DNA and RNA method. Moreover, some variants that were identified by DNA were absent or poorly expressed in RNA, and important promoter variants such as TERT are not identified by said test.18
Jug, et al. (2018) evaluated the performances of ThyroSeq and Afirma GEC within the context of ultrasonographic features and with the incorporation of the “noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) nomenclature.” The ultrasonographic pattern evaluations were derived from the 2015 American Thyroid Association guidelines. A total of 304 cases were evaluated, with 119 resections. All cases that met criteria for NIFTP were considered high-risk by both tests. However, when these NIFTP cases were moved from the malignant to the non-malignant category, the positive predictive value of ThyroSeq dropped from 42.9% to 14.3% and Afirma’s dropped from 30.1% to 25.3%.19
Endo, et al. (2019) compared Afirma’s GEC to its Gene Sequencing Classifier (GSC), using cytologically indeterminate nodules, 343 GEC-tested nodules and 164 GSC-tested nodules were identified. The GSC was found to have a “statistically significant higher benign call rate (76.2% vs. 48.1%), PPV (60.0% vs. 33.3%), and specificity (94.3% vs. 61.4%).” The authors noted that the improvement was statistically significant for Bethesda III and IV nodules. The GSC benign call rate was significantly higher in Hürthle cell changes (88.8% vs 25.7%). Out of the indeterminate nodules 52.5% went to surgery when using the GEC compared to only 17.6% when using the GSC.20 Vuong, et al. (2021) completed a meta-analysis of seven studies comparing the clinical impact and diagnostic performance of Afirma’s GEC and GSC. Similarly, this study showed that GSC had a higher benign cell rate, particularly in Hürthle cell predominated nodules, as well as a lower resection rate and higher risk of malignancy. The authors conclude that “the specificity (43.0% vs 25.1%; P = .003) and PPV (63.1% vs 41.6%; P = .004) of Afirma GSC were significantly improved while it still maintained a high sensitivity (94.3%) and a high (90.0%)” compared to the GEC.21
Kim, et al. (2023) compared the performance of Afirma GSC and ThyroSeq v3 in 372 indeterminate thyroid nodules. The study reported that of the 173 nodules tested with Afirma GSC, 51% were classified as benign, 42% as suspicious, and 7% were insufficient for analysis. Among the 89 nodules classified as benign by Afirma GSC, 20% were surgically resected, with no malignancies found on histopathology. Of the 72 nodules classified as suspicious, 88% were resected, revealing 36 malignancies on histopathology. The sensitivity of Afirma GSC in detecting true positive cases and correctly identifying malignant nodules was reported to be 100%. The specificity was 77%.13
RosettaGX Reveal
The RosettaGX Reveal test from Rosetta Genomics is a micro-RNA-based diagnostic test that evaluates indeterminate thyroid nodules. The test measures 24 sequences of miRNA through quantitative RT-PCR, as well as a medullary carcinoma marker (hsa-miR-375). Each sample is classified as benign or suspicious for malignancy.
The test has a claimed negative predictive value of 99%, a 98% sensitivity, and a 78% specificity from a sample of 150 in which three pathologists all agreed on the evaluation. Out of the 189 total samples, the negative predictive value was 91%, sensitivity was 85%, and specificity was 72%. The nodule sizes were >0.5 cm. The authors concluded that this test may be able to differentiate between malignant and benign from a previously evaluated indeterminate sample.22 This test has been discontinued.
NeoGenomics
NeoGenomics offers a “NeoTYPE” thyroid profile, intended for evaluation of “fine needle aspirates of thyroid nodules that are indeterminate or suspicious on cytology.” NeoGenomics states that FISH detects mutations and other gene rearrangements, and that BRAF mutation V600E is associated with poor prognosis of papillary thyroid carcinoma. The test analyzes 32 biomarkers through a combination of next-generation sequencing (NGS), FISH, and IHC as listed below: “AKT1, ALK, ARID1A, ATM, BRAF, CDKN2A, CTNNB1, ERBB2, ERBB4, HRAS, KRAS, MEN1, MET, Microsatellite Instability (MSI), NF1, NF2, NRAS, PIK3CA, PTEN, RET, SMAD4, SMO, SRC, TERT Promoter, TP53, TSC1, TSC2, Tumor Mutation Burden (TMB)”, MET and RET by FISH, PD-L1, and Pan-TRK by IHC.23
Clinical Utility and Validity
A study focusing on detection of BRAF, RAS, RET/PTC, and PAX8/PPARg mutations found that detection of any mutation resulted in a risk of histologic malignancy of 88%, 87%, and 95% for samples of Bethesda categories III, IV, and V, respectively, 967 samples were taken, and 87 mutations were found. The risk of cancer in mutation-negative samples was 6%, 14%, and 28%, respectively. Unfortunately, there was also a 14% false negative rate, limiting the usefulness of this panel.5,24
Additional point mutations (including TERT, TP53, and others), as well as gene fusions that occur in thyroid cancer were found in another study using a next-generation sequencing (NGS) assay. A total of 143 samples with a classification of follicular neoplasm or suspicious for follicular neoplasm were assessed, and the NGS panel “ThyroSeq v2” was used, which tests for point mutations in 13 genes and 42 types of gene fusions. Out of these samples 104 were found to be benign with the other 39 sample results being malignant. Overall, the NGS panel performed at a negative predictive value of 96%, a positive predictive value of 83%, 90% sensitivity, 93% specificity, and 92% accuracy for FN/SFN nodules.25
Another method that has been used is assessment of mRNA expression through a gene expression classifier. This is the basis of the Afirma genomic sequencing classifier, which identifies markers for features, such as medullary thyroid cancer, as well as distinguishes between Hürthle cell neoplasms from non-neoplastic Hürthle cell neoplasms. The second version of this test was assessed with 191 indeterminate samples. The sensitivity and specificity were 91% and 68%, respectively, and the negative and positive predictive values were 96% and 47%, respectively.5,26 Afirma has produced a similar gene expression classifier “Xpression Atlas” that can be used to assess B-III to B-VI category neoplasms. Xpression Atlas evaluates a total of 761 gene variants and 130 fusion pairs.5,27
The above methods may be combined to better evaluate neoplasms, for instance, a miRNA gene expression classifier using both mutation analysis and miRNA expression. Labourier, et al. (2015) evaluated a diagnostic algorithm that combined mutation analysis and miRNA expression to assess preoperative FNAs. This algorithm contained 17 validated gene alterations using the miRInform thyroid test and a 10-miRNA gene expression classifier. 109 samples of either AUS/FLUS or FN/SFN were evaluated with this combined algorithm, and this algorithm correctly identified 64% of malignant samples and 98% of benign ones. The sensitivity and specificity were 89% and 85%, respectively. For AUS/FLUS and FN/SFN, the negative predictive value was 97% and 91%, and the positive predictive value for malignancy was 68% and 82%, respectively.5,28
No single methodology has achieved clinical utility to reliably resolve all indeterminate cytology, and thus several professional organizations, including the American Association of Clinical Endocrinologists (AACE), the American Thyroid Association , and the National Comprehensive Cancer Network , have published guidelines for the evaluation of thyroid nodules, all of which endorse a similar multistep strategy suggesting molecular markers can be of use when cytology is indeterminate, yet acknowledging its current limitations.29-32
Banizs and Silverman (2019) evaluated the utility of “combined mutation analysis and microRNA classification in reclassifying cancer risk of cytologically indeterminate thyroid nodules.” A three-tiered microRNA threshold was determined based on nodules with known disease status, and an expected rate of malignancy calculated from mutation analysis and the microRNA approach was validated in an independent cohort of “atypia of undetermined significance or follicular lesion of undetermined significance (AUS/FLUS) and follicular neoplasm or suspicious for follicular neoplasm (FN/SFN) nodules with surgically derived outcomes.” From there, 2685 patients were included in the intended analysis. 82% of these samples lacked mutations, with BRAF, PIK3CA, PAX8/PPARg, and RET/PTC mutations all comprising 2% or less. The maximum expected risk of malignancy in these nodules without mutation was 9% for AUS/FLUS nodules and 17% for FN/SFN nodules, but with positive microRNA status, these rates increased to 36% and 54% respectively. RAS mutations occurred in 15% of mutations, and the expected malignancy rates in nodules with RAS or PAX8/PPARg mutations was 49% for AUS/FLUS nodules and 65% for FN/SFN nodules. With positive microRNA status, these rates increased to 85% and 91%, respectively.33
National Comprehensive Cancer Network (NCCN)
NCCN guidelines for thyroid carcinoma state that molecular diagnostic testing to detect individual mutations (such as BRAF V600E or RET/PTC) or pattern recognition approaches using molecular classifiers may be useful in the evaluating indeterminate FNA samples to assist in management decisions. NCCN states molecular diagnostics may be used to reclassify indeterminate lesions such as AUS/FLUS. Molecular markers should be interpreted within the context of each patient. The NCCN states that molecular testing may be considered to drive treatment decisions, and some mutations may have prognostic importance.
When FNA results suggest atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS, Bethesda III) and there is not a high clinical and/or radiographic suspicion of malignancy, the NCCN recommends that one considers repeat FNA with second opinion pathology, diagnostic lobectomy, molecular diagnostics, or node surveillance as recommended by the ATA or TI-RADS as treatment. However, they remind readers that “Molecular markers should be interpreted with caution and in the context of clinical, radiographic, and cytologic features of each individual patient. If molecular diagnostics are technically inadequate or not done, then repeat FNA.”32
The NCCN further clarifies that “The choice of the precise molecular test depends on the cytology and the clinical question being asked. Indeterminate groups include: 1) follicular or oncocytic neoplasms; and 2) AUS/FLUS.” The NCCN panel recommends molecular diagnostic testing for evaluating FNA results that are suspicious for follicular cell neoplasms or AUS/FLUS (category 2A), including oncocytic carcinoma, as studies historically do not perform well on Hürthle neoplasms. However, the NCCN has acknowledged promising studies for assessing oncocytic carcinoma neoplasms, with both the Afirma and ThyroSeq v3 tests providing better evaluations than their predecessors. The NCCN notes that molecular diagnostic testing may include individual mutation analysis or a multigene assay such as a gene expression classifier. Molecular markers may also help with treatment decisions or with eligibility in clinical trials.
However, they also note that “The diagnosis of follicular carcinoma or oncocytic carcinoma requires evidence of either vascular or capsular invasion, which cannot be determined by FNA. Molecular diagnostics may be useful to allow reclassification of follicular lesions (i.e., follicular neoplasm, AUS, FLUS) as either more or less likely to be benign or malignant based on the genetic profile. If molecular testing suggests papillary thyroid carcinoma, especially in the case of BRAF V600E, see PAP 1 Given the challenges of cytology to explicitly diagnose MTC in limited samples, molecular tests may be used to identify them. If molecular testing, in conjunction with clinical and ultrasound features, predicts a risk of malignancy comparable to the risk of malignancy seen with a benign FNA cytology (approximately 5% or less), consider nodule surveillance. Molecular markers should be interpreted with caution and in the context of clinical, radiographic, and cytologic features of each individual patient. If molecular diagnostics are technically inadequate or not done, then repeat FNA.”32
For individuals with or suspected with having papillary carcinoma, the NCCN recommends the following procedures as part of the diagnostic workup: thyroid and neck ultrasound (including central and lateral compartments), if not previously done, CT/MRI with contrast for locally advanced disease or vocal cord paresis, Consider assessment of vocal cord mobility (ultrasound, mirror indirect laryngoscopy, or fiberoptic laryngoscopy), FNA for suspicious lateral neck nodes.32 FNA is important to consider in this case as “Tg washout is useful in diagnosis of lymph node metastases and recommended if cytology is negative.”32
The NCCN further notes that active surveillance may be an option for patients whose molecular diagnostics demonstrate a risk of malignancy under 5% and that the predictive value of molecular diagnostics may be influenced by pre-test probability of disease based on various FNA cytology groups. The NCCN states that molecular diagnostic testing may be useful for follicular cell carcinomas and diagnosing NIFTP [noninvasive follicular thyroid neoplasm with papillary-like nuclear features], although current tests for assessing NIFTP have not been validated. Molecular testing is not recommended for diagnosing anaplastic thyroid carcinoma. Finally, the NCCN highlights that the diagnostic utility of molecular diagnostics in pediatric patients is still unclear because most of the published literature is on adult patients with thyroid nodules.32
American Thyroid Association (ATA)
The 2015 ATA guidelines on the management of adult patients with thyroid nodules and differentiated thyroid cancer make the following recommendations on the use of molecular markers:
- “If molecular testing is being considered, patients should be counseled regarding the potential benefits and limitations of testing and about the possible uncertainties in the therapeutic and long-term clinical implications of results.” (Strong recommendation; low-quality evidence)
- “If intended for clinical use, molecular testing should be performed in Clinical Laboratory Improvement Amendments/College of American Pathologists (CLIA/CAP)-certified molecular laboratories, or the international equivalent, because reported quality assurance practices may be superior compared to other settings.” (Strong recommendation; low-quality evidence)
- “For nodules with AUS/FLUS cytology, after consideration of worrisome clinical and sonographic features, investigations such as repeat FNA or molecular testing may be used to supplement malignancy risk assessment in lieu of proceeding directly with a strategy of either surveillance or diagnostic surgery. Informed patient preference and feasibility should be considered in clinical decision-making.” (Weak recommendation; moderate-quality evidence)
- “Diagnostic surgical excision is the long-established standard of care for the management of FN/SFN cytology nodules. However, after consideration of clinical and sonographic features, molecular testing may be used to supplement malignancy risk assessment data in lieu of proceeding directly with surgery. Informed patient preference and feasibility should be considered in clinical decision-making.” (Weak recommendation; moderate-quality evidence)
- In general, only nodules >1 cm should be evaluated as they have a greater chance to become a clinically significant cancer. However, there are some cases where nodules <1 cm may be evaluated due to other clinical symptoms. The ATA states that “attempts to diagnose and treat all such small thyroid cancers in an effort to prevent exceedingly rare outcomes is deemed to cause more harm than good.”
- “If the nodule is benign on cytology, further immediate diagnostic studies or treatment are not required.”
- “Each nodule that is >1 cm carries an independent risk of malignancy and therefore multiple nodules may require FNA.”29
The guidelines also state that "there is currently no single optimal molecular test that can definitively rule in or rule out malignancy in all cases of indeterminate cytology, and long-term outcome data proving clinical utility are needed.”29
The ATA Guidelines Task Force on Pediatric Thyroid Cancer have developed unique guidelines for children and adolescents with thyroid tumors. They have presented 34 recommendations including recommendations on molecular markers testing and nodules. The ATA panel recommended the pediatric age to be limited to a patient that is < 18 years of age to more accurately define the impact of the physiologic changes of growth and development on tumor behavior. These guidelines note that a size criterion is more difficult in children as age affects volume greatly and size of the nodule is not predictive of malignancy. Overall, studies focusing on molecular diagnostics in children have not been validated and so cannot be recommended at this time.34
The 2021 ATA Guidelines for Management of Patients with Anaplastic Thyroid Cancer notes that FNA cytology can be an important diagnostic tool for ATC diagnosis but recommends a parallel core biopsy to obtain sufficient material for molecular testing and accurate diagnosis.30,35 Other notable recommendations are reported below.
“RECOMMENDATION 2
Every effort should be made to establish a diagnosis via biopsy before proceeding with surgical resection, as surgical resection may be inappropriate.
Strength of Recommendation: Strong
Quality of Evidence: Low”
Moreover, the ATA asserts that “Generous sampling of the tumor, including its advancing edge, is indicated and necessary to fully characterize the neoplasm and increase the probability of finding any coexistent carcinoma and/or other pathologic findings. Additional or repeat immunohistochemical analysis should be performed as needed.” Consequently, surgical pathology evaluation is indicated in the below situations.
“RECOMMENDATION 3
Routine surgical pathology evaluation of resection specimens should focus on confirming a definitive diagnosis of ATC, documenting the extent of disease, and defining the presence of any coexisting DTC and/or other pathologies. The proportion of tumor that represents ATC should also be documented.
Strength of Recommendation: Strong
Quality of Evidence: Low
RECOMMENDATION 4
Once ATC diagnosis is considered, assessment of BRAFV600E mutation should be expeditiously performed by IHC and confirmed/expeditiously assessed by molecular testing.
Strength of Recommendation: Strong
Quality of Evidence: Moderate”
Regarding the timing of molecular profiling, the ATA offers the following recommendation:
“RECOMMENDATION 5
Molecular profiling should be performed at the time of ATC diagnosis to inform decisions related to the use of targeted therapies, especially as there are now FDA-approved mutation-specific therapies in this context.
Strength of Recommendation: Strong
Quality of Evidence: Moderate.
For initial evaluations, “There are a number of preoperative staging procedures of importance in ATC.” The ATA propounds the below procedures.
“RECOMMENDATION 6
Initial radiological tumor staging should include cross-sectional imaging, in particular, CT neck, chest, abdomen, and pelvis with contrast (or MRI), and, if available, FDG PET/CT. Contrast-enhanced imaging of the brain (MRI preferred) should also be performed, if clinically indicated.
Strength of Recommendation: Strong
Quality of Evidence: Moderate.”35
American Academy of Pediatrics (AAP)
The AAP endorsed the guidelines of the American Thyroid Association Guidelines Task Force on Pediatric Thyroid Cancer (as presented in Francis, et al. (2015)) in a publication released in 2018.36
A 2025 study from Boston Children's Thyroid Center, a collaborator of the AAP, reviewed 26 years of data from 496 pediatric patients on pediatric thyroid FNA procedures. The study found that FNA is generally safe and well-tolerated in children, with minimal pain and complications. They recommend the continued use of FNA in pediatric patients.37
American Association of Clinical Endocrinologists (AACE), American College of Endocrinology and Associazione Medici Endocrinologi
These joint guidelines recommend the following:
- “Molecular testing should be considered to complement, not replace cytologic evaluation, and only if the results are expected to influence clinical management. As a general rule, molecular testing is not recommended in nodules with established benign or malignant cytologic characteristics.”
- FNA is recommended for high ultrasound (US) risk lesions of ≥10 mm, intermediate risk lesions of >20 mm, and low risk lesions >20 mm and growing or with a risk history.
- “FNA is not recommended for nodules that are functional on scintigraphy.”
- Repeat FNA is recommended in benign nodules with suspicious clinical or US findings, a nondiagnostic initial FNA on a solid nodule, and nodules that become symptomatic or increase in volume by 50%. Pregnancy does not affect cytologic diagnostic criteria. Routine repeat FNA is generally not necessary.
- Nodules with a major diameter of <5 mm should be monitored instead of biopsied.
- Consider the detection of BRAF and RET/PTC and possibly PAX8/PPARG and RAS mutations if available.
- There is no stance on gene expression classifiers for indeterminate nodules, due to insufficient evidence and limited follow-up.
- There is also insufficient evidence to take any stance on mutation testing to guide surgery decisions, except on mutations with a PPV approaching 100% for papillary thyroid carcinoma such as BRAFV600E.31
National Institute for Care and Health Excellence (NICE)
The guidelines concerning the use of fine needle aspiration in the assessment and management of thyroid cancer are reported below.
"Management options based on ultrasound results
1.2.9 Offer fine needle aspiration cytology (FNAC) to people who meet the threshold using an established system for grading ultrasound appearance.
1.2.10 Consider FNAC or active surveillance for people who do not meet the threshold for FNAC on ultrasound grading alone if there are other reasons for clinical concern.”38
“FNAC testing
Performing and reporting FNAC
1.2.11 See the recommendation on using ultrasound [1.2.9, 1.2.10] guidance when performing FNAC in the NICE guideline on thyroid disease.
1.2.12 Use liquid-based cytology, direct smear or both when processing FNAC samples.
1.2.13 Use the Royal College of Pathologists modification of the British Thyroid Association (BTA) reporting system to report cytology results.
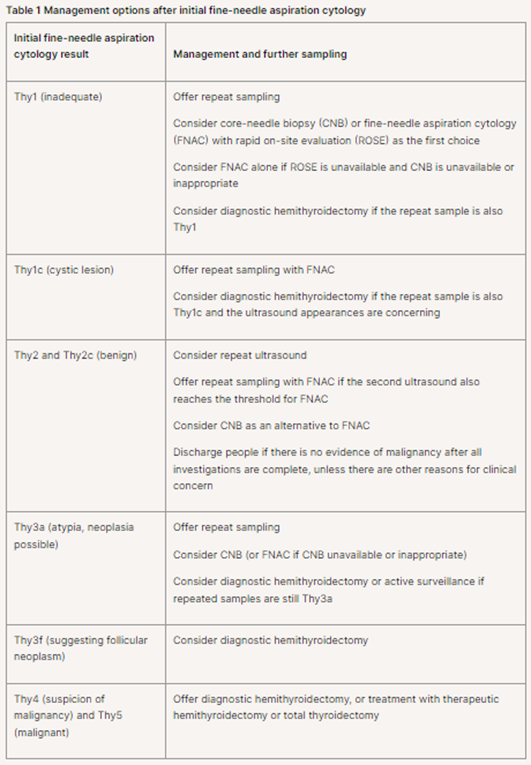
1.2.14 Consider rapid on-site evaluation of FNAC adequacy rates to improve the diagnostic yield of samples if the Thy1 (inadequate) rate for the center or individual clinicians is higher than 15% (when Thy1c is excluded).”38
“Management and further sampling after initial FNAC
“1.2.15 Use the initial FNAC results to determine further management and sampling options, as shown in table 1.”
Table 1 is captured below. Repeat sampling with FNAC is recommended for Thy1c (cystic lesion).
Diagnosing, managing and monitoring thyroid enlargement with normal thyroid function
“1.9.3 When making decisions about whether to offer fine needle aspiration cytology, use an established system for grading ultrasound appearance that takes into account:
- echogenicity
- microcalcifications
- border
- shape in transverse plane
- internal vascularity
- lymphadenopathy.
1.9.4 Reports of ultrasound findings should:
- specify which grading system has been used for the assessment.
- include information on the features in recommendation 1.9.3 and
- provide an overall assessment of malignancy, and
- confirm that both lobes have been assessed, and
- document assessment of cervical lymph nodes.
1.9.5 Use ultrasound guidance when performing fine needle aspiration cytology.”38
European Thyroid Association (ETA)
The ETA states that molecular testing for BRAF, RET/PTC and possibly PAX8, PPARG, and RAS mutations may be considered for cytologically indeterminate lesions. The search for molecular markers in B-II class lesions is not recommended, although one member of the panel did not agree with this non-recommendation. A GEC test cannot be recommended to exclude malignancy to replace diagnostic surgery or close surveillance, although one member did not agree with this non-recommendation. The targeted NGS approach is considered the most promising, with larger panels potentially becoming a rule-in and rule-out test if >95% negative predictive value can be reached. The ETA notes that molecular diagnostics may reduce completion thyroidectomies or other surgeries due to clearer assessments of an indeterminate lesion.39
In their 2023 Clinical Practice Guidelines for thyroid nodule management, the ETA stated that
- “Combine clinical factors, laboratory evaluation, and US risk stratification when defining the indication for FNA, in a shared decision with the patient (Ungraded good practice statement. Agreement: 9/9 (100%); round: 1)
- US guidance and use of either capillary action or suction is recommended when performing thyroid nodule FNA (Strength of recommendation: 1; quality of evidence: ØØØO. Agreement: 9/9 (100%); round: 1)
- FNA indication should be based on the following size cut-offs:
- EU-TIRADS 5: >10 mm
- EU-TIRADS 4: >15 mm
- EU-TIRADS 3: >20 mm
(Strength of recommendation: 2; quality of evidence: ØOOO. Agreement: 9/9 (100%); round: 1)
- Repeat FNA should be considered in case of a first non-diagnostic sample (excluding the solitary cyst), a Bethesda class III cytology, discrepancy between US risk score (i.e. high risk) and cytological findings (i.e. benign cytology), and significant nodule growthc (Strength of recommendation: 1; quality of evidence: ØØØO. Agreement: 8/9 (88.9%); round: 1)
- FNA is recommended in suspicious lymph nodes, with thyroglobulin or calcitonin washout dependent on phenotype (Strength of recommendation: 1; quality of evidence: ØØØO. Agreement: 9/9 (100%); round: 1)
- Core-needle biopsy should not be used as a first-line tool to assess thyroid nodules after US but could be considered a second-line procedure for specific conditions (Strength of recommendation: 1; quality of evidence: ØØOO. Agreement: 8/9 (88.9%); round: 1).”40
Regarding cytopathology-based management, the ETA recommend that practitioners “Correlate the cytological diagnosis with clinical, ultrasound and laboratory results (Ungraded good practice statement. Agreement: 9/9 (100%); round: 1).” Moreover, “Clinical surveillance of benign thyroid nodules which do not require therapeutic intervention, should be followed up according to the management schedule” below.

(Extracted from Table 1 of Durante, et al. (2023).)
However, the ETA also states that “Molecular testing may be considered in cytologically indeterminate nodules, if available (Strength of recommendation: 1; quality of evidence: ØØØO. Agreement: 9/9 (100%); round: 1).”
Regarding the use of non-ultrasound imaging modalities, it is recommended that FNA be avoided in specific instances: “Thyroid scintigraphy should be performed when serum TSH is subnormal in order to diagnose functioning nodules and/or multinodularity, avoid FNA and determine eligibility for RAI as an alternative to surgery (Strength of recommendation: 1; quality of evidence: ØØØO. Agreement: 9/9 (100%); round: 1).”40
European Society for Medical Oncology (ESMO)
The ESMO remarks that preoperative FNA for cytology is “not required” for nodules 1 cm or smaller. ESMO states that FNA diagnosis “can be facilitated” by assessment of malignancy markers and molecular alterations. Specifically designed gene panels are “reportedly” useful for identifying malignancy with indeterminate samples.41
References:
1. Ross D. Diagnostic approach to and treatment of thyroid nodules. Updated Jul 08, 2024. https://www.uptodate.com/contents/diagnostic-approach-to-and-treatment-of-thyroid-nodules
2. Nikiforov YE, Yip L, Nikiforova MN. New strategies in diagnosing cancer in thyroid nodules: impact of molecular markers. Clinical cancer research : an official journal of the American Association for Cancer Research. May 01 2013;19(9):2283-8. doi:10.1158/1078-0432.ccr-12-1253
3. Jackson S, Kumar G, Banizs AB, et al. Incremental utility of expanded mutation panel when used in combination with microRNA classification in indeterminate thyroid nodules. Diagnostic Cytopathology. 2020;48(1):43-52. doi:10.1002/dc.24328
4. Ali SZ, Baloch ZW, Cochand-Priollet B, Schmitt FC, Vielh P, VanderLaan PA. The 2023 Bethesda System for reporting thyroid cytopathology. J Am Soc Cytopathol. Sep-Oct 2023;12(5):319-325. doi:10.1016/j.jasc.2023.05.005
5. Douglas R. Evaluation and management of thyroid nodules with indeterminate cytology. Updated Aug 29, 2024. https://www.uptodate.com/contents/evaluation-and-management-of-thyroid-nodules-with-indeterminate-cytology
6. Cibas ES, Ali SZ. The Bethesda System for Reporting Thyroid Cytopathology. American Journal of Clinical Pathology. 2009;132(5):658-665. doi:10.1309/AJCPPHLWMI3JV4LA
7. Hodak SP, Rosenthal DS. Information for clinicians: commercially available molecular diagnosis testing in the evaluation of thyroid nodule fine-needle aspiration specimens. Thyroid : official journal of the American Thyroid Association. Feb 2013;23(2):131-4. doi:10.1089/thy.2012.0320
8. Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet (London, England). Mar 23 2013;381(9871):1058-69. doi:10.1016/s0140-6736(13)60109-9
9. Interpace. microRNA Signatures and Mutational Analysis Can Provide Confidence and Convenience. https://thygenext-thyramir.com/
10. Lupo MA, Walts AE, Sistrunk JW, et al. Multiplatform molecular test performance in indeterminate thyroid nodules. Diagnostic Cytopathology. 2020;48(12):1254-1264. doi:10.1002/dc.24564
11. Ciarletto AM, Narick C, Malchoff CD, et al. Analytical and clinical validation of pairwise microRNA expression analysis to identify medullary thyroid cancer in thyroid fine-needle aspiration samples. Cancer Cytopathol. Mar 2021;129(3):239-249. doi:10.1002/cncy.22365
12. ThyroSeq. ThyroSeq® GC - the most comprehensive NGS test for thyroid nodules available. https://www.thyroseq.com/physicians/test-description/
13. Kim NE, Raghunathan RS, Hughes EG, et al. Bethesda III and IV Thyroid Nodules Managed Nonoperatively After Molecular Testing With Afirma GSC or Thyroseq v3. The Journal of Clinical Endocrinology & Metabolism. 2023;108(9):e698-e703. doi:10.1210/clinem/dgad181
14. Afirma. Afirma® Genomic Sequencing Classifier. https://www.afirma.com/physicians/why-afirma/#a1
15. Wei S, Veloski C, Sharda P, Ehya H. Performance of the Afirma genomic sequencing classifier versus gene expression classifier: An institutional experience. Cancer Cytopathology. 2019/11/01 2019;127(11):720-724. doi:10.1002/cncy.22188
16. Randolph GW, Sosa JA, Hao Y, et al. Preoperative Identification of Medullary Thyroid Carcinoma (MTC): Clinical Validation of the Afirma MTC RNA-Sequencing Classifier. Thyroid : official journal of the American Thyroid Association. Sep 2022;32(9):1069-1076. doi:10.1089/thy.2022.0189
17. Angell TE, Wirth LJ, Cabanillas ME, et al. Analytical and Clinical Validation of Expressed Variants and Fusions From the Whole Transcriptome of Thyroid FNA Samples. 10.3389/fendo.2019.00612. Frontiers in Endocrinology. 2019;10:612.
18. Krane JF, Cibas ES, Endo M, et al. The Afirma Xpression Atlas for thyroid nodules and thyroid cancer metastases: Insights to inform clinical decision-making from a fine-needle aspiration sample. Cancer Cytopathology. 2020;128(7):452-459. doi:10.1002/cncy.22300
19. Jug RC, Datto MB, Jiang XS. Molecular testing for indeterminate thyroid nodules: Performance of the Afirma gene expression classifier and ThyroSeq panel. Cancer Cytopathol. Jul 2018;126(7):471-480. doi:10.1002/cncy.21993
20. Endo M, Nabhan F, Porter K, et al. Afirma Gene Sequencing Classifier Compared with Gene Expression Classifier in Indeterminate Thyroid Nodules. Thyroid : official journal of the American Thyroid Association. Aug 2019;29(8):1115-1124. doi:10.1089/thy.2018.0733
21. Vuong HG, Nguyen TPX, Hassell LA, Jung CK. Diagnostic performances of the Afirma Gene Sequencing Classifier in comparison with the Gene Expression Classifier: A meta-analysis. Cancer Cytopathol. Mar 2021;129(3):182-189. doi:10.1002/cncy.22332
22. Lithwick-Yanai G, Dromi N, Shtabsky A, et al. Multicentre validation of a microRNA-based assay for diagnosing indeterminate thyroid nodules utilising fine needle aspirate smears. Journal of clinical pathology. Jun 2017;70(6):500-507. doi:10.1136/jclinpath-2016-204089
23. NeoGenomics. NeoTYPE® Thyroid Profile. Updated January 22, 2025. https://www.neogenomics.com/providers/test/NTG-TYPX-02CX/neotype-thyroid-profile/
24. Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. The Journal of clinical endocrinology and metabolism. Nov 2011;96(11):3390-7. doi:10.1210/jc.2011-1469
25. Nikiforov YE, Carty SE, Chiosea SI, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. Dec 01 2014;120(23):3627-34. doi:10.1002/cncr.29038
26. Patel KN, Angell TE, Babiarz J, et al. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules. JAMA Surgery. 2018;153(9):817-824. doi:10.1001/jamasurg.2018.1153
27. Afirma. NEW Afirma GSC + Xpression Atlas. https://www.afirma.com/physicians/practice-resources/#a6
28. Labourier E, Shifrin A, Busseniers AE, et al. Molecular Testing for miRNA, mRNA, and DNA on Fine-Needle Aspiration Improves the Preoperative Diagnosis of Thyroid Nodules With Indeterminate Cytology. The Journal of clinical endocrinology and metabolism. Jul 2015;100(7):2743-50. doi:10.1210/jc.2015-1158
29. Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. Jan 2016;26(1):1-133. doi:10.1089/thy.2015.0020
30. ATA. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. 2021;31(3):337-386. doi:10.1089/thy.2020.0944
31. Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules--2016 Update. Endocr Pract. May 2016;22(5):622-39. doi:10.4158/EP161208.GL
32. NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Thyroid Carcinoma s Version 5.2024. Updated January 15, 2025. https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf
33. Banizs AB, Silverman JF. The utility of combined mutation analysis and microRNA classification in reclassifying cancer risk of cytologically indeterminate thyroid nodules. Diagn Cytopathol. Apr 2019;47(4):268-274. doi:10.1002/dc.24087
34. Francis GL, Waguespack SG, Bauer AJ, et al. Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. Jul 1 2015;25(7):716-59. doi:10.1089/thy.2014.0460
35. Bible KC, Kebebew E, Brierley J, et al. 2021 American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. Mar 2021;31(3):337-386. doi:10.1089/thy.2020.0944
36. AAP. Management Guidelines for Children With Thyroid Nodules and Differentiated Thyroid Cancer. Pediatrics. Dec 2018;142(6)doi:10.1542/peds.2018-3063
37. Mazzantini S, Cherella CE, Graziano C, et al. Thyroid Fine-Needle Aspiration Is Safe and Well-Tolerated in Children. Thyroid®. 2025;35(1):111-114. doi:10.1089/thy.2024.0549
38. NICE. Thyroid cancer: assessment and management. NICE. Updated October 2023. Accessed 2024, https://www.nice.org.uk/guidance/ng230/chapter/Recommendations#assessment-and-diagnosis
39. Paschke R, Cantara S, Crescenzi A, Jarzab B, Musholt TJ, Sobrinho Simoes M. European Thyroid Association Guidelines regarding Thyroid Nodule Molecular Fine-Needle Aspiration Cytology Diagnostics. European thyroid journal. Jul 2017;6(3):115-129. doi:10.1159/000468519
40. Durante C, Hegedüs L, Czarniecka A, et al. 2023 European Thyroid Association Clinical Practice Guidelines for thyroid nodule management. European thyroid journal. Oct 1 2023;12(5)doi:10.1530/etj-23-0067
41. Filetti S, Durante C, Hartl D, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology. 2019;30(12):1856-1883. doi:10.1093/annonc/mdz400 %J Annals of Oncology
Coding Section
| Code |
Number |
Description |
| CPT |
81210 |
BRAF (B-Raf proto-oncogene, serine/threonine kinase) (e.g., colon cancer, melanoma), gene analysis, V600 variant(s) |
|
|
81401 |
Molecular pathology procedure, Level 2 (e.g., 2-10 SNPs, 1 methylated variant, or 1 somatic variant [typically using nonsequencing target variant analysis], or detection of a dynamic mutation disorder/triplet repeat) |
|
|
81445 |
Solid organ neoplasm, genomic sequence analysis panel, 5 – 50 genes, interrogation for sequence variants and copy number variants or rearrangements, if performed; DNA analysis or combined DNA and RNA analysis |
|
|
81455 |
Solid organ or hematolymphoid neoplasm or disorder, 51 or greater genes, genomic sequence analysis panel, interrogation for sequence variants and copy number variants or rearrangements, or isoform expression or mRNA expression levels, if performed; DNA analysis or combined DNA and RNA analysis |
|
|
81479 |
Unlisted molecular pathology procedure |
|
|
81546 |
Oncology (thyroid), mRNA, gene expression analysis of 10,196 genes, utilizing fine needle aspirate, algorithm reported as a categorical result (e.g., benign or suspicious) |
|
|
81599 |
Unlisted multianalyte assay with algorithmic analysis |
|
|
0018U |
Oncology (thyroid), microRNA profiling by RT-PCR of 10 microRNA sequences, utilizing fine needle aspirate, algorithm reported as a positive or negative result for moderate to high risk of malignancy |
|
|
0026U |
Oncology (thyroid), DNA and mRNA of 112 genes, next-generation sequencing, fine needle aspirate of thyroid nodule, algorithmic analysis reported as a categorical result ("Positive, high probability of malignancy" or "Negative, low probability of malignancy") |
|
|
0204U (Code deleted effective 07/01/2024) |
Oncology (thyroid), mRNA, gene expression analysis of 593 genes (including BRAF, RAS, RET, PAX8, and NTRK) for sequence variants and rearrangements, utilizing fine needle aspirate, reported as detected or not detected |
|
|
0245U |
Oncology (thyroid), mutation analysis of 10 genes and 37 RNA fusions and expression of 4 mRNA markers using next-generation sequencing, fine needle aspirate, report includes associated risk of malignancy expressed as a percentage |
|
|
0287U |
Oncology (thyroid), DNA and mRNA, next-generation sequencing analysis of 112 genes, fine needle aspirate or formalin-fixed paraffin-embedded (FFPE) tissue, algorithmic prediction of cancer recurrence, reported as a categorical risk result (low, intermediate, high) |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2014 Forward
| 04/10/2025 | Annual review, no change to policy intent. Updating description, note, rationale, guidelines/recommendations, and references. |
| 06/24/2024 | Interim review to indicate PLA code 0204U is deleted effective 07012024. |
| 04/18/2024 | Annual review, no change to policy intent. Also updating table of terminology, rationale, references and verbiage of multiple CPT codes. |
| 09/11/2023 | Updating Coding section. Adding code 0362U effective 10/01/2023. No other changes made. |
| 04/18/2023 | Annual review, no change to policy intent, but, policy is being rewritten for clarity and consistency. Also updating description, table of terminology, rationale and references. |
| 04/28/2022 |
Annual review, removing note regarding 50 genes allowed for testing. Also updating rationale, references and coding. Adding table of terminology. |
| 01/31/2022 |
Interim review, adding end date 01012022 to code 0208U. |
| 04/20/2021 |
Annual review, no change to policy intent, but, verbiage clarified for specificity. Also updating rationale, references coding and policy number. |
| 12/17/2020 |
Updating Coding Section with 2021 codes. |
| 04/20/2020 |
Annual review, expanding medical necessity criteria and reformatting for clarity. |
| 04/04/2019 |
Annual review, no change to policy intent. Updating coding. |
| 08/04/2018 |
Corrected formatting. No other changes. |
| 05.01/2018 |
Interim review, rewriting entire policy to address medical necessity for BRAF, V600E, RET/PTC, RAS, PAX8/PPAR. Clarification of medically necessary, not medically necessary and investigational uses for this testing. |
| 02/05/2018 |
Interim review adding medical necessity criteria for ThyroSeqv2, ThyraMIR microRNA/ThyGenX, Afirma BRAF after Afirma Gene Expression Classifier or Afirma MTC after Afirma Gene Expression Classifier. Also updating background, description, guidelines, rationale and references. |
| 04/25/2017 |
Updated category to Laboratory. No other changes. |
| 04/17/2017 |
Annual review, no change to policy intent. |
| 01/04/2017 |
Annual review, no change to policy intent. |
| 1/21/2016 |
Updated review date |
| 01/13/2016 |
Interim review, major revision to policy including medical necessity criteria. This testing was previously considered investigational. Also updated rationale and references. |
| 01/05/2016 |
Added CPT Code 81479 to Coding Section. No other changes made to policy. |
| 12/1/2015 |
Updating CPT codes for 2016. No change to policy intent. |
| 02/19/2015 |
Annual review, no change to policy intent. Updated rationale and references. Added guidelines and coding. |
| 02/17/2014 |
Annual review, updated title, description, regulatory status, rationale, summary and references. Added policy language that states "The use of a gene expression classifier in fine-needle aspirates of the thyroid that are cytologically considered to be indeterminate, atypical or suspicious for malignancy is considered to be investigational." |