Parathyroid Hormone, Phosphorus, Calcium and Magnesium Testing - CAM 217
Description
Parathyroid hormone (PTH), along with calcitriol and fibroblast growth factor 23 (FGF23), regulate calcium and phosphate homeostasis. PTH modulates the serum ionized calcium concentration by stimulating kidney reabsorption of calcium as well as increasing bone resorption within minutes of PTH secretion. Primary hyperparathyroidism presents itself with hypercalcemia and elevated PTH levels and is typically caused by parathyroid adenoma or hyperplasia. Secondary hyperparathyroidism is seen “in patients with kidney failure who have increased secretion of PTH [and] is related not only to gland hyperplasia and enlargement but also to reduced expression of CaSRs [calcium-sensing receptors] and, perhaps, its downstream signaling elements.”1
Calcium is an essential metal found in its biologically relevant divalent cation (Ca2+) form in vivo. It is involved in many important biological processes, including cell signaling, signal transduction, and muscle contraction. Only 45% of the plasma calcium is in the ionized form (or ‘free’ form), which is the physiologically active form, while the rest is bound to albumin or complexed to anions, such as phosphate or citrate.2 Both total calcium and ionized calcium can be tested from a blood sample. Occasionally, calcium concentration is determined from a 24-hour urine sample.3
Phosphorus is typically used in its oxidized phosphate polyatomic ionic form (PO43-) in vivo and is an important functional group in all classes of biomolecules—carbohydrates, proteins, lipids, and nucleic acids. The cytosol uses a phosphate-based buffer to maintain pH homeostasis. Plasma phosphorus can be in either organic or inorganic form, but the inorganic phosphates are regulated by hormones, primarily PTH. Typically, phosphate/phosphorus testing is performed on a blood sample but it can also be performed on a urine sample.4
Magnesium, like calcium, in vivo is in its divalent cation (Mg2+) form. It is involved in many enzymatic mechanisms as well as structural functions for both proteins and nucleic acids. Magnesium is required for maintenance of bone health as well as proper nerve conduction, muscle contraction, and energy production. Currently, magnesium is tested from a blood sample or less frequently from a 24-hour urine sample. Due to the large amounts of magnesium that is filtered and the degree of reabsorption and secretion in urine levels, “magnesium levels in the urine do not correlate with either the amount of magnesium ingested or the magnesium status in the body.”
Policy
Application of coverage criteria is dependent upon an individual’s benefit coverage at the time of the request.
- Serum intact parathyroid (PTH) testing is considered MEDICALLY NECESSARY in any of the following situations:
- For individuals with abnormal calcium levels.
- One time testing for the diagnosis of hypoparathyroidism for individuals with signs of hypoparathyroidism (see Note 1).
- For individuals with osteoporosis or low bone mass.
- For individuals who have undergone parathyroidectomy.
- One test every year for individuals diagnosed with hyperparathyroidism and who have not undergone parathyroidectomy.
- At the following frequency for individuals with chronic kidney disease (CKD):
- For individuals with Grade 3 CKD: One test every twelve months.
- For individuals with Grade 4 or Grade 5 CKD: One test every three months.
- One time testing for individuals with multiple endocrine neoplasia type 2A (MEN2A) or familial medullary thyroid carcinoma.
- At the following frequency for individuals who have pseudohypoparathyroidism or related disorders (see Note 2):
- For individuals who are less than 18 years of age, one test every three months.
- For individuals who are 18 years of age or older, one test every year.
- Serum intact parathyroid (PTH) testing to screen for asymptomatic hyperparathyroidism is considered NOT MEDICALLY NECESSARY.
- For individuals presenting for a wellness visit or a general exam without abnormal findings, the following tests is considered NOT MEDICALLY NECESSARY:
- Serum, blood, or fecal magnesium testing.
- Serum phosphorus or phosphate testing.
- Urine phosphorus or phosphate testing.
- Serum total calcium, serum ionized calcium, or urine calcium testing.
- Serum parathyroid hormone testing.
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of an individual’s illness.
- Testing serum for truncated parathyroid hormone metabolites (e.g., amino-terminal and carboxy-terminal fragments) is considered NOT MEDICALLY NECESSARY.
NOTES:
Note 1: Signs of hypoparathyroidism:6
- Hypocalcemia
- Elevated serum phosphorus
- Low calcitriol
- Hypercalciuria
- Abnormal magnesium
Note 2: Conditions of pseudohypoparathyroidism and related disorders:7
- Pseudohypoparathyroidism Type 1A (PHP1A)—due to maternal loss of function mutation at the GNAS coding sequence
- Pseudohypoparathyroidism Type 1B (PHP1B)—due to methylation defect at the GNAS coding sequence
- Pseudopseudohypoparathyroidism (PPHP)—due to paternal loss of function mutation at the GNAS coding sequence
- Progressive Osseous Heteroplasia (POH)—due to paternal loss of function mutation at the GNAS coding sequence
- Acrodysostosis (ACRDYS1)—due to mutation in PRKAR1A
- Acrodysostosis (ACRDYS2)—due to mutation in PDE4D
Table of Terminology
| Term |
Definition |
| 25[OH]D |
25-hydroxy-vitamin D |
| AACC |
American Association for Clinical Chemistry |
| AACE |
American Association of Clinical Endocrinologists |
| AAES |
American Association of Endocrine Surgeons |
| AAP |
American Academy of Paediatrics |
| ACE |
American College of Endocrinology |
| ACRDYS1 |
Acrodysostosis type 1 |
| ACRDYS2 |
Acrodysostosis type 2 |
| AGA |
American Gastroenterological Association |
| AHA |
American Heart Association |
| AHO |
Albright hereditary osteodystrophy |
| ALL |
Acute lymphoblastic leukemia |
| ALP |
Alkaline phosphatase |
| ASA |
American Society of Anesthesiologists |
| ASCO |
American Society of Clinical Oncology |
| ASMBS |
American Society for Metabolic & Bariatric Surgery |
| ATA |
American Thyroid Association |
| ATLL |
Adult T-Cell leukemia/lymphoma |
| AUA |
American Urological Association |
| BRUE |
Brief resolved unexplained events |
| BUN |
Blood urea nitrogen |
| Ca |
Calcium |
| Ca2+ |
Calcium in its biologically relevant divalent cation form |
| CAD |
Coronary artery disease |
| CaSRs |
Calcium-sensitive receptors |
| CBC |
Complete blood count |
| CCO |
Cancer Care Ontario |
| CKD |
Chronic kidney disease |
| CLIA ’88 |
Clinical Laboratory Improvement Amendments of 1988 |
| CLL |
Chronic lymphocytic leukemia |
| CMS |
Centers for Medicare and Medicaid |
| CRPC |
Castration resistant prostate cancer |
| CUP |
Cancer of unknown primary |
| CVs |
Coefficients of variation |
| EDTA |
Ethylenediaminetetraacetic acid |
| eGFR |
Estimated glomerular filtration rate |
| ESCC |
Esophageal squamous cell carcinomas |
| FDA |
Food and Drug Administration |
| FGF |
Fibroblast growth factor |
| FGF23 |
Fibroblast growth factor 23 |
| GCTB |
Giant cell tumor of bone |
| GD |
Graves' disease |
| GFR |
Glomerular filtration rate |
| GNAS |
Guanine nucleotide binding protein, alpha Stimulating activity polypeptide |
| GPCRs |
G-protein coupled receptors |
| HCHC |
Hypocalciuric hypercalcemia |
| HIV |
Human immunodeficiency virus |
| HPFS |
Health professionals follow-up study |
| HPT |
Hyperparathyroidism (non-specific) |
| ICSI |
Institute for Clinical Systems Improvement |
| IFCC |
International Federation of Clinical Chemistry |
| IPM |
Intraoperative pseudoparathyroidism monitoring |
| iPTH |
Intact parathyroid hormone |
| KDIGO |
Kidney Disease Improving Global Outcomes |
| LDH |
Lactate dehydrogenase |
| LDTs |
Laboratory-developed tests |
| LFTs |
Liver function tests |
| MEN1 |
Multiple endocrine neoplasia type 1 |
| MEN2 |
Multiple endocrine neoplasia type 2 |
| MEN2A |
Multiple endocrine neoplasia type 2A |
| Mg |
Magnesium |
| Mg2+ |
Magnesium in its divalent cation form |
| MS |
Multiple sclerosis |
| NBA |
National Blood Authority |
| NCCMH |
National Collaborating Centre for Mental Health |
| NCCN |
National Comprehensive Cancer Network |
| NGC |
National Guideline Clearinghouse |
| NGS |
Next generation sequencing |
| NHS I |
Nurses’ Health Study I |
| NHS II |
Nurses’ Health Study II |
| NICE |
National Institute for Health and Care Excellence |
| OMA |
Obesity Medical Association |
| PDE4D |
Phosphodiesterase 4D |
| PHP |
Pseudoparathyroidism |
| PHP1A |
Pseudohypoparathyroidism type 1A |
| PHP1B |
Pseudohypoparathyroidism type 1B |
| pHPT |
Primary hyperparathyroidism |
| PHPT |
Primary hyperparathyroidism |
| PO43- |
Phosphorus in its oxidized phosphate polyatomic ionic form |
| POH |
Progressive osseous heteroplasia |
| PPHP |
Pseudopseudohypoparathyroidism |
| PTH |
Parathyroid hormone |
| PTH-rP |
Parathyroid hormone-related protein |
| rhPTH |
Recombinant human parathyroid hormone |
| SLL |
Small lymphocytic lymphoma |
| SLCA |
Systemic light chain amyloidosis |
| SOGC |
Society of Obstetricians and Gynaecologists of Canada |
| TLS |
Tumour lysis syndrome |
| TOS |
The Obesity Society |
| TSH |
Thyroid stimulating hormone |
| UA |
Urine analysis |
| WHO |
World Health Organization |
Rationale
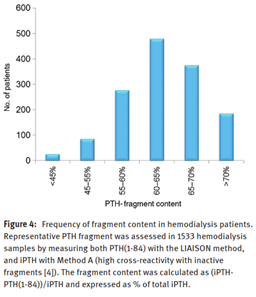
Parathyroid hormone (also called parathormone or PTH) is a peptide hormone that is 84 amino acids long when first secreted by the parathyroid gland. It has a biological half-life of approximately two to four minutes before being proteolyzed into smaller fragments. These truncated fragments can comprise as much as 95% of the total circulating immunoreactive PTH. PTH is released whenever the serum ionized calcium concentration decreases as detected by the calcium-sensing receptor. Once released, PTH can increase serum calcium concentrations by increasing bone resorption as well as decreasing renal calcium excretion and increasing calcitriol production1. The bar graph figure below is taken from Valcour et al. (2018), and shows the predominance of the truncated fragments circulating in hemodialysis patients. These truncated PTH peptides can interfere with many serum PTH testing methods.8,9
Both PTH and PTH-related protein analogues may assist in osteoporosis therapy as each play a key role in bone metabolism; it is widely accepted that PTH is an important regulator of calcium homeostasis in the body10. PTH has been FDA approved as an anabolic treatment for osteoporosis.10 The PTH hormone analog teriparatide is known to stimulate bone remodeling, increase the mineral density in the hip and spine bones, and reduce the risk of fractures in postmenopausal osteoporotic women.11 Some patients with elevated PTH levels also exhibit vitamin D deficiency, while others do not; however, elevated PTH levels seem to affect both postural stability and muscle function.12 More research needs to be completed in this area.
Hyperparathyroidism is characterized by high serum phosphate levels, low serum calcium levels, and abnormal PTH levels; this disease is rare and can be managed with active vitamin D and calcium supplements.13 Researchers have noted that treatment with recombinant human parathyroid hormone (rhPTH) may be a good treatment option for patients with hyperparathyroidism who cannot maintain normal urinary and serum calcium levels.13
The amount of calcium in the bloodstream is monitored by the parathyroid glands. These glands release PTH, which increases blood calcium levels. Magnesium modulates parathyroid hormone secretion; particularly, high magnesium levels increase PTH when the parathyroid glands are exposed to low calcium levels.14 Serum calcium may be high due to primary hyperthyroidism and malignancy, or low due to hypothyroidism or renal failure; abnormal serum calcium levels may lead to bone abnormalities or issues in the kidneys, the parathyroid gland, or the gastrointestinal tract.15
Hypercalcemia is defined as high calcium levels in the blood stream; this may be caused by hyperparathyroidism, drugs, malignancy, or granulomatous disorders.16 Hypercalcemia caused by PTH is the most common cause of primary hyperthyroidism. “Algorithms for diagnosis of PTH related hypercalcaemia require assessment of a 24-h urinary calcium and creatinine excretion to calculate calcium/creatinine clearance ratio and radiological investigations including ultrasound scan and 99mTc-sestamibi-SPECT/CT”.16
Serum phosphate homeostasis is principally regulated by the work of PTH and FGF23 via vitamin D. PTH primarily regulates calcium metabolism with secondary effects on phosphate whereas FGF23 is the opposite. Primary hyperparathyroidism (PHPT) often results in hypophosphatemia, but PTH resistance either due to surgical ablation or autoimmune disorders can cause hyperphosphatemia. PTH increases the release of phosphate from bone and the absorption of intestinal phosphate, but it increases the renal excretion of phosphate.17
Typically, serum magnesium homeostasis is regulated by the kidneys. However, large increases in PTH increases bone resorption and can also affect the loop of Henle, the location of magnesium reabsorption in the kidneys, to decrease magnesium excretion.18 Certain types of tumor cells, including esophageal squamous cell carcinomas (ESCC) release a parathyroid hormone-related protein (PTH-rP). A study by Konishi et al. (2018) has demonstrated that PTH and PTH-rP affect magnesium homeostasis in ESCC receiving cisplatin therapy. The researchers found that “intravenous Mg supplementation therefore conferred protective effects against cisplatin-induced nephrotoxicity in patients with ESCC. Furthermore, increases in PTH or PTH-rP may have influenced the extent of nephrotoxicity”.19 Hernandez-Becerra et al. (2020) found that, in rats, a calcium deficiency due to diet results in less magnesium identified in bones, including an apparent lower bone mineral density and a thinner cortical bone and trabecular bone porosity.
Analytical Validity
The International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) established a Working Group to research how pre-analytical conditions affected the measurement of PTH in blood samples.21 This extensive review covered everything from circadian rhythms and how time of day affected clinical validity to storage conditions and seasonal changes. The research included data from 83 different studies. The authors note that the inclusion of EDTA to the sample will increase the stability to at least 72 hours for plasma samples and to 24 hours for serum samples. PTH concentrations in the summer are lower than in the winter months for patients in the Northern hemisphere, and it is noted that “PTH has a circadian rhythm characterized by a nocturnal acrophase and mid-morning nadir”.21 The data was found to be contradictory concerning the validity of results obtained from frozen samples regardless of whether the sample was stored at -20◦C or -80◦C. PTH concentrations were also considerably higher in central blood as compared to peripheral blood (median values of 24.3 pmol/L versus 15.3 pmol/L, respectively). It is recommended that “blood samples for PTH measurement should be taken into tubes containing EDTA, ideally between 10:00 [a.m.] and 16:00 [p.m.], and plasma separated within 24 h of venipuncture. Plasma samples should be stored at four degrees Celsius and analysed within 72 h of venipuncture. Particular regard must be paid to the venipuncture site when interpreting PTH concentration. Further research is required to clarify the suitability of freezing samples prior to PTH measurement”.21
The IFCC Working Group on PTH also investigated how to improve PTH testing, especially with regards to the need for common references and standards. “Recent increases in understanding of the complex pathophysiology of CKD [chronic kidney disease], which involves calcium, phosphate and magnesium balance, and is also influenced by vitamin D status and fibroblast growth factor (FGF)-23 production, should facilitate such improvement. Development of evidence-based recommendations about how best to use PTH is limited by considerable method-related variation in results, of up to five-fold, as well as by lack of clarity about which PTH metabolites these methods recognize. This makes it difficult to compare PTH results from different studies and to develop common reference intervals and/or decision levels for treatment.”22 The graph below (taken from 22,23) compares the differences between various available PTH assays observed within a single patient specimen.
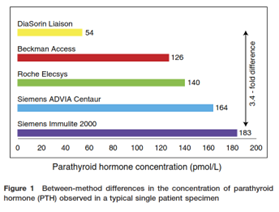
The study by Almond, et al. (2012) shows that up to 4.2-fold differences can occur between these testing methods, and “these differences were sufficient to have treatment implications for 79% of the patients in the pilot study.” The 2017 IFCC study shows that “within-laboratory within-method coefficients of variation (CVs) <10%”; however, “between-laboratory between-method CVs are generally >20%.”22
Bensalah, et al. (2018) analyzed the differences in PTH serum measurement between the Roche Cobas e411® (which uses a chemiluminescent sandwich enzyme immunoassay) and the Abbott Architect ci8200® (which uses a chemiluminescent microparticle immunoassay); this study included 252 patients. The two techniques were compared by the Bland-Altman difference diagram. “In conclusion, our study shows a great discrepancy between the results of the PTH assay on the Architect ci8200 versus the Cobas e411,” suggesting that currently marketed kits need to be evaluated further.24
Clinical Utility and Validity
Since serum PTH testing can be complicated by the presence of proteolytic fragments as well as a brief biological half-life of mere minutes, Valcour, et al. (2018) evaluated the efficacy of the LIAISON 1-84 PTH test, a third-generation serum test, as compared to other intact testing methods. This study was conducted at three different locations throughout the United States.
Each test site recruited fifteen patients, and the patients were equally divided into three groups—healthy patients, primary hyperparathyroid patients, and hemodialysis patients. A minimum of nine samples were collected from each patient. Each test’s efficacy was also evaluated concerning how the sample was collected (plasma EDTA, unspun plasma EDTA, and serum separator) as well as how storage time at room temperature affected results (up to 72 hours). Two different standards were used—the WHO 95/646 international standard and the synthetic Bachem PTH (1-84) standard. Both the second- and third-generation intact PTH test were consistent with the standards up to 72 hours; however, the “serum is significantly less stable than plasma when samples are stored at room temperature for 72 h regardless of platform, even when separated from the clot by centrifugation within 1 h.”8 The mean percent change from baseline ranged from 96%-107% for the LIAISON 1-84 test except for the serum at 72 h, which had a mean of 82%. Likewise, the second-generation mean percent change from baseline ranged from 95%-108% except for the serum at 72 h, which again was 82%. The authors conclude that the “LIAISON 1-84 PTH assay is accurate and reliably measures the biologically active PTH molecule in plasma or serum stored at room temperature for up [to] 27 and 24 h, respectively.”8
A study at the Cleveland Clinic of more than 2.7 million patients’ electronic medical records was published in 2013 looking at the prevalence of PHPT, both symptomatic and asymptomatic, and the correlation with serum calcium testing. Of the records obtained, two percent had serum calcium levels >10.5 mg/dL, and 1.3% of the total patient population had previously been diagnosed with PHPT. Only 32% of the patients who had not been previously diagnosed with either hypercalcemia, PHPT, or had undergone a parathyroidectomy had recorded PTH values in their medical records. “Patients with calcium of 11.1 – 11.5 mg/dL were most likely to have PHPT (55%). Patients with calcium >12 mg/dL were most likely to have PTH measured. Of hypercalcemic patients, 67% never had PTH obtained, …. It is estimated that 43% of hypercalcemic patients are likely to have PHPT….”; The authors conclude, “it is crucial to evaluate even mild hypercalcemia, because 43% of these patients have PHPT. PHPT is underdiagnosed and undertreated.”25
In 1975, Pak and colleagues published results of a urine test they developed to diagnose hypercalciuria.26 Since then, 24-hour urinary calcium testing is a common clinical practice, especially in monitoring kidney health, with reference values of <250 mg/24 hours for males and <200 mg/24 hours for females.27 A comprehensive study by Curhan, et al. (2001) investigated the 24-hour urine concentrations of calcium, magnesium, and phosphorus along with several other analytes. Calcium and magnesium were quantified by atomic absorption spectroscopy whereas phosphorus was measured using a Cobas centrifugal analyzer. Samples were collected from over 1000 patients who were already taking part in three large-scale ongoing cohort studies—NHS I (Nurses’ Health Study I), NHS II, and HPFS (Health Professionals Follow-Up Study). Neither magnesium nor phosphate was significant in any of the three cohorts between the patients with kidney stones and the controls; however, the urine calcium concentration was significantly elevated (p ≤ 0.01) in two of the three cohorts. One cohort, though, had 27% of the patients in the control group exhibiting hypercalciuria and only 33% of the experimental group exhibiting hypercalciuria. Conclusions state that “the traditional definitions of normal 24-hour urine values need to be reassessed, as a substantial proportion of controls would be defined as abnormal.”28
Serum magnesium testing can be used in monitoring preeclampsia and hypermagnesemia. The reference values are age-dependent, but levels greater than nine mg/dL can be life-threatening.29 The evidence of causation or the use of serum magnesium in predicting preeclampsia have been inconclusive. A study by Kreepala, et al. (2018) has proposed the use of serum total magnesium and ionized magnesium levels to develop a magnesium-based equation for screening of preeclampsia. This study involved 84 pregnant women including 20 controls. The remaining 64 had been diagnosed with preeclampsia after the 20th week of pregnancy. The authors determined that the serum ionized magnesium levels were “significantly lower in preeclampsia group (23.95 ± 4.7% vs. 26.28 ± 2.3%, p = .04).” The equation that was developed has an “area of ROC for predictive accuracy of the model [of] 0.77 (p <.001). [The] ROC suggested that the score of 0.27 would be a threshold for screening preeclampsia with 70% sensitivity and 81% specificity.” Kreepala, et al. (2018) suggest “blood testing on total and ionized magnesium concentrations as well as calculation of ionized magnesium fraction in addition to routine antenatal care for better screening of the disease.”
Serum magnesium levels have been identified to play a role in other disorders as well. Low serum magnesium levels have recently been associated with a greater coronary artery disease (CAD) risk.31,32 A total of 14446 participants were followed for one year in a large meta-analysis study. The researchers concluded that “low circulating Mg was associated with higher CAD risk than was higher Mg”; however, it was not determined whether magnesium concentration manipulation could assist in the prevention of coronary artery disease.32 Mancuso, et al. (2020) conducted a separate study that further validated the association between serum magnesium and CAD. They concluded that Mg2+ could be used to assess subclinical cardiovascular organ damage, including increased carotid artery intima-media thickness and left ventricular mass index in “hypertensive patients with asymptomatic subclinical vascular atherosclerotic disease and with higher cardiovascular risk.” Higher serum Mg2+ concentrations could possibly be protective against progression of CAD as well.
Sri-Ganeshan, et al. (2022) conducted a retrospective observational study in a single emergency department, measuring calcium (in 1426 patients), magnesium (in 1296 patients), and phosphate (in 1099 patients). Part of the study involved a clinical tool that analyzed patient electrolyte risk factors, (that is, abnormal calcium, magnesium, and phosphate levels). The “over-testing” of electrolytes in an ER setting is an area of concern, noted the authors. Researchers hypothesized they could use a decision-making tool to determine clinical factors associated with low and high levels of each electrolyte, then only test patients who met the criteria. The authors postulated that “patients without a single risk factor in the tool are unlikely to have clinically significant abnormal Ca, Mg or PO4 levels and do not require [further] testing.” After analyzing results, the authors found very high NPVs for both Ca and Mg, “If Ca and Mg had only been measured in patients with a risk factor for an abnormality, a very small proportion patients (approximately 1%) would not have been identified.” However, the authors noted that the use of such a clinical decision-making tool appeared to be less robust when it came to phosphate testing.34
2016 American Association of Endocrine Surgeons (AAES)
The AAES released guidelines concerning primary hyperparathyroidism (pHPT) in 2016. With respect to laboratory testing, in Recommendation 1-1, these guidelines state, “The biochemical evaluation of suspected pHPT should include serum total calcium, PTH, creatinine, and 25-hydroxyvitamin D levels (strong recommendation; moderate-quality evidence).” The AAES also addresses differentiating between pHPT and suspected “familial hypocalciuric hypercalcemia, which is an autosomal dominant disorder of the ren
pHPT.” In Recommendation 1-2 (strong recommendation; moderate-quality evidence), “a 24-hour urine measurement of calcium and creatinine should be considered in patients undergoing evaluation for possible pHPT…. Familial hypocalciuric hypercalcemia should be considered in patients with long-standing hypercalcemia, urinary calcium levels less than 10 mg/24 hours, and a calcium to creatinine clearance ratio less than 0.01.”
The AAES also address the use of intraoperative PTH monitoring (IPM). Recommendation 6-1: “When image-guided focused parathyroidectomy is planned, IPM is suggested to avoid higher operative failure rates (strong recommendation; moderate-quality evidence).” However, a strong recommendation with low-quality evidence to recommendation 6-2 was provided: “Surgeons who use IPM should use a sampling protocol that is reliable in the local environment and should be familiar with the interpretation of PTH decay dynamics.” The frequency of testing either calcium or PTH post-operatively is not given, but the AAES mentions these recommendations in several comments concerning the monitoring or measuring calcium and/or PTH levels or determining post-operative hyper-/hypoparathyroidism (Recommendation 14-7, Recommendation 15-1a, Recommendation 15-1b, Recommendation 15-3, and Recommendation 15-4). It is also stated that the definition of a success versus failure of operation is when levels are compared six months post-operation.35
“The assessment of cure and complications (prolonged hypoparathyroidism, permanent RLN paralysis) requires evaluation for at least 6 months. Longitudinal testing should include calcium, PTH, and 25-hydroxyvitamin D levels. Achieving normal vitamin D levels postoperatively will help absorption of calcium and normalization of PTH levels . . .”
“Recommendation 14-7: At 6 months, surgeons individually or in conjunction with the multidisciplinary care team should assess postparathyroidectomy patients for cure and evidence of long-term complications (strong recommendation; low-quality evidence).”
“Although there is no role for routine PTH measurement in the normocalcemic patient in the immediate postoperative period, failure to normalize PTH levels at 6 months or longer can signify early operative failure. In normocalcemic pHPT, the PTH level must also normalize to indicate cure. In patients with inherited forms of pHPT, a different end point of care pertains.”
“Recommendation 15-1a: Cure after parathyroidectomy is defined as the reestablishment of normal calcium homeostasis lasting a minimum of 6 months (strong recommendation; high-quality evidence).
Recommendation 15-1b: Patients with normocalcemic pHPT who have persistently elevated PTH levels after parathyroidectomy should be evaluated and treated for causes of secondary HPT and, if none are present, monitored for recurrent disease (strong recommendation; low-quality evidence).”
“Recommendation 15-3: In normocalcemic pHPT, the definition of cure must include normal calcium and PTH levels more than 6 months after surgery (insufficient evidence).”
“Recommendation 15-4: Persistent pHPT should be defined as a failure to achieve normocalcemia within 6 months of parathyroidectomy. Recurrent pHPT is defined by recurrence of hypercalcemia after a normocalcemic interval at more than 6 months after parathyroidectomy (strong recommendation; high-quality evidence).”35
2018 First International Consensus Statement on Pseudohypoparathyroidism and Related Disorders
An international consortium of representatives from across Europe and North America released their first international consensus statement, including extensive guidelines and recommendations, concerning pseudohypoparathyroidism and related disorders in 2018. These disorders have a wide array of phenotypes but are due to impaired cell signaling cascades of G-protein coupled receptors (GPCRs). Pseudohypoparathyroidism can be classified as either type 1A or 1B (PHP1A and PHP1B, respectively), depending on the type of defect in the GNAS coding sequence. Pseudopseudohypoparathyroidism (PPHP) and progressive osseous heteroplasia (POH) are caused by a paternal loss of function defect to GNAS. Acrodysostosis is classified as either type one (ACRDYS1) or type two (ACRDYS2) due to mutations in either PRKAR1A or PDE4D, respectively. PTH resistance can be negligible in infancy but typically increases with age.
In recommendation 1.3 (A+++), the guidelines list the clinical and biochemical major criteria for diagnosing PHP and related disorders, including “PTH resistance, and/or subcutaneous ossifications that can include deeper ossifications, and/or early-onset (before two years of age) obesity associated with TSH resistance or with one of the above, and/or AHO [Albright hereditary osteodystrophy] alone” regardless of family history. In recommendation 1.6 (A+++), “The definition of PTH resistance is as follows: [1] The association of hypocalcaemia, hyperphosphataemia and elevated serum levels of PTH in the absence of vitamin D deficiency and when magnesium levels and renal function are normal. [2] PTH resistance in the context of PHP and related disorders should be suspected when PTH is at, or above, the upper limit of normal, in the presence of normal calcifediol levels and elevated serum levels of phosphorus, even in the absence of overt hypocalcaemia. PTH resistance and consequent changes in serum levels of calcium, phosphorus and PTH can be variable, and repeated testing might be required.” In all cases, genetic counseling is recommended.
In recommendation 3.2, the measurement of serum PTH, calcium, phosphorus, and calcifediol are recommended “at diagnosis or before initiation of treatment”; moreover, “measurement of PTH, calcium and phosphorus should be performed regularly (every six months in children and at least yearly in adults) with the exception of patients carrying either a GNAS mutation on the paternal allele or a PDE4D mutation in whom, apart from diagnosis, routine assessment is not necessary. Monitoring of serum levels of calcium should be more frequent in symptomatic individuals, during acute phases of growth, during acute illness and during pregnancy and breastfeeding….” For patients undergoing vitamin D therapy, they stress as part of recommendation 3.4 (A++) that serum phosphate be monitored. Concerning patients undergoing treatment for PTH resistance, in recommendation 3.5 (A++), the guidelines state that “levels of PTH, calcium and phosphorus should be monitored every six months in asymptomatic patients and more frequently when clinically indicated.” In recommendation 3.26 (A+), the routine measurement of calcitonin is not recommended.7
2020 European Network on Pseudohypoparathyroidism (EuroPHPnet)
The EuroPHPnet published its “Recommendations for Diagnosis and Treatment of Pseudohypoparathyroidism and Related Disorders: An Updated Practice Tool for Physicians and Patients.” In these guidelines, the EuroPHPnet noted that “PTH resistance is the hallmark of PHP [pseudohypoparathyroidism], found in 45-80% of patients,” and symptoms of PTH resistance should not be ignored and “screening and follow-up of PTH resistance should include measurement of PTH, 25-OH vitamin D, calcium, and phosphate every three to six months in children and at least yearly in adults.” However, the frequency of monitoring is also contingent on whether the individual is symptomatic or not, in acute phases of growth, experiencing intercurrent illness, pregnancy, or is breastfeeding. In the case of pregnant women with hypocalcemia and/or hypothyroidism, they “should be monitored following the international guidelines for any pregnancy associated with these disturbances” and their newborns “should be evaluated for the presence of skin ossifications and levels of TSH, calcium, and phosphorous.”36
International Workshop on the Evaluation and Management of Primary Hyperparathyroidism
The Fifth International Workshop on the Evaluation and Management of Primary Hyperparathyroidism convened in 2022 and published their guidelines as a consensus statement in The Journal of Bone and Mineral Research.
For the diagnosis of asymptomatic hypercalcemic PHPT “where biochemical screening is commonly performed, most patients with PHPT come to clinical attention when hypercalcemia is found unexpectedly in the context of an investigation of an unrelated problem or simply upon routine testing. If the PTH level is also found to be high, or even in the normal range, the most likely diagnosis is asymptomatic hypercalcemic PHPT.”
When diagnosing normocalcemic PHPT, “PTH levels may be measured in the evaluation of medical conditions such as osteoporosis, low bone mass, or nephrolithiasis. Normocalcemic PHPT (NPHPT) is characterized by persistently normal albumin-adjusted total and ionized serum calcium levels, accompanied by elevated levels of PTH on at least two consecutive measurements, over a three month to six month period.”37
The workshop also included a section on genetic testing where they note that testing for mutations in one of 10 genes can facilitate the diagnosis of a syndromic or nonsyndromic form of PHPT, which helps in clinical management and treatment. Specifically, they note that “genetic testing helps to identify family members who may or may not be at risk. Genetic counseling and evaluation, thus, should be considered for patients <30 years with PHPT, those with multigland disease by history or imaging, those with a family history of hypercalcemia or syndromic diseases such as MEN1, MEN2A, MEN4, or HPT-JT syndrome, and in patients with atypical parathyroid adenoma and parathyroid carcinoma.”37
The 2014 workshop established guidelines for monitoring patients with asymptomatic primary hyperparathyroidism (PHPT) and recommended annual testing of serum calcium. A formula was given to determine corrected calcium concentration, which is recommended (rather than using free calcium), since “most centers do not have sufficient capabilities to rely upon an ionized, free calcium concentration”:
Corrected [Ca] = [total serum calcium in mg/dL + 0.8*(4.0 - patient’s serum albumin in g/dL)]
Recommendations for evaluating asymptomatic PHPT are shown in Table 3 shown below although the guidelines do state that “this evaluation is for PHPT, not to distinguish between PHPT and other causes of hypercalcemia.” This table includes calcium (both serum and 24-hour urine testing) and phosphate testing.
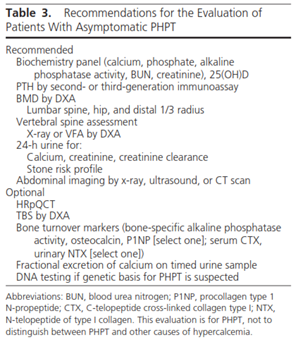
In their algorithm for monitoring patients with normocalcemic PHPT, both annual calcium and PTH testing are included; however, there is no mention of the method of calcium testing (i.e. serum versus 24-hour urine testing) or phosphate testing.38
National Comprehensive Cancer Network (NCCN)
The NCCN addresses PTH, calcium, phosphate, and magnesium testing in several different guidelines.
Neuroendocrine & Adrenal Tumors:39 The NCCN continues to assert that “Primary hyperparathyroidism associated with parathyroid adenomas is the most common manifestation of MEN1 [Multiple endocrine neoplasia, type 1]. Measurement of serum calcium levels is recommended if hyperparathyroidism is suspected. If calcium levels are elevated, parathyroid hormone (PTH) and 25-OH vitamin D levels should be checked.” With respect to the surveillance of MEN1-associated parathyroid tumors, “The panel recommends annual calcium and serum PTH levels to screen for parathyroid tumors. If calcium levels rise, 25-OH vitamin D should be measured and imaging with neck ultrasound and/or parathyroid sestamibi with SPECT scan (SPECT-CT preferred) or 4D-CT should be performed.” Similarly, for the evaluation of patients with Multiple Endocrine Neoplasia Type 2 (MEN2), “serum calcium levels should be measured. If it is found to be elevated, PTH and 25-OH vitamin D levels should be measured. A neck ultrasound, sestamibi scan with SPECT, or 4D-CT scan can also be performed as appropriate.”
Acute Lymphoblastic Leukemia (ALL):40 As part of the initial workup for ALL patients, they recommend “a tumor lysis syndrome (TLS) panel (including measurements for serum lactate dehydrogenase [LDH], uric acid, potassium, phosphates, and calcium).” In the section concerning the supportive care of ALL in steroid management, they guidelines state to “obtain vitamin D and calcium status and replete as needed” and monitor possible osteonecrosis/avascular necrosis associated as a potential long-term side effect of corticosteroids. Likewise, the NCCN later stated “To monitor patients for risks of developing symptomatic osteonecrosis, routine measurements for vitamin D and calcium levels should be obtained and periodic radiographic evaluation (using plain films or MRI [magnetic resonance imaging]) should be considered.”
Systemic Light Chain Amyloidosis:41 As part of the initial diagnostic workup, in the section titled “Laboratory evaluation for SLCA,” the NCCN recommends testing “serum BUN [blood urea nitrogen]/creatinine, electrolytes, albumin, calcium, serum uric acid, serum LDH, and beta-2 microglobulin. A 24 hour urine for total protein, urine protein electrophoresis (UPEP), and urine immunofixation electrophoresis (UIFE).”
Bone Cancer:42 In the section concerning the workup of Giant Cell Tumor of Bone (GCTB), a rare benign tumor, the guidelines state that “brown tumor of hyperparathyroidism should be considered as a differential diagnosis; routine evaluation of serum calcium, phosphate, and parathyroid hormone levels can help exclude this diagnosis.” Moreover, prior to treatment of bone lesions, it is recommended that “Laboratory studies, such as complete blood count (CBC), comprehensive metabolic panel (CMP) with calcium to assess for hypercalcemia, lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) should be done prior to initiation of treatment.”
Breast Cancer:43 In general, in monitoring metastatic disease, “laboratory tests such as alkaline phosphatase, liver function, blood counts, and calcium…” are to be included to help aid the clinician in determining “the effectiveness of treatment and the acceptability of toxicity.”
Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (CLL/SLL):44 Small-molecule inhibitors, such as Venetoclax, are possible therapies for CLL/SLL. Tumor Lysis Syndrome (TLS) is a possible side effect of such treatment. In the section on supportive care for CLL/SLL, they note that “patients with bulky lymph nodes, progressive disease after small-molecular inhibitor therapy, and receiving chemotherapy, venetoclax, lenalidomide, obintuzumab are considered to be at high-risk for TLS.” NCCN further states that laboratory hallmarks of TLS include high potassium, uric acid, phosphorous, lactate dehydrogenase, and low calcium. In Venetoclax therapy, particularly, they state to “evaluate blood chemistries (potassium, uric acid, phosphorus, calcium, and creatinine); review in real time.” The table below (adapted from the guideline) depicts the blood chemistry monitoring as recommended:
| Blood Chemistry Monitoring (potassium uric acid, phosphorus, calcium and creatinine) |
|
| Low Tumor Burden |
|
| Outpatient setting |
Pre-dose, 6 – 8 hours, 24 hours at first dose of 20 mg and 50 mg Pre-dose at subsequent ramp-up doses |
| Medium Tumor Burden |
|
| Outpatient setting |
Pre-dose, 6 – 8 hours, 24 hours at first dose of 20mg and 50mg Pre-dose at subsequent ramp-up doses Consider hospitalization for patients with CrCl < 80 mL/min at first dose of 20 mg and 50 mg |
| High Tumor Burden |
|
| In hospital setting |
At first dose of 20 and 50 mg Pre-dose 4 hrs 8 hrs 12 hrs 24 hrs |
| Outpatient setting (for subsequent ramp-up doses) |
Pre-dose 6 – 8 hrs 24 hrs |
Esophageal and Esophagogastric Junction Cancers:45 In the section on principles of survivorship under Management of Long-Term Sequelae of Disease or Treatment, they say to “consider monitoring vitamin B, folic acid, vitamin D, and calcium levels.” Moreover, following esophagectomy, long-term calcium deficiency is common along with deficiencies in vitamin B12, folic acid, and vitamin D.
Kidney Cancer:46 The NCCN uses serum calcium levels as a predictor of short survival used to select patients for temsirolimus, as well as a prognostic factor [i.e. “calcium > upper limit of normal (Normal: 8.5-10.2 mg/dL)”] in accordance with the Memorial Sloan Kettering Cancer Center Prognostic Model and the International Metastatic Renal Cell Carcinoma Database Consortium Criteria. The guidelines do not state how frequently serum calcium should be tested or if it is solely for use at diagnosis. However, the guidelines recommend that laboratory evaluation for patients with renal cell carcinoma typically present with a suspicious mass involving the kidney may include a metabolic panel consisting of “corrected calcium, serum creatinine, liver function studies, and urinalysis.”
Multiple Myeloma:47 In the initial diagnostic workup for multiple myeloma, the NCCN recommends testing “serum BUN/creatinine, electrolytes, liver function tests, albumin, calcium, serum uric acid, serum LDH, and beta-2 microglobulin.” As follow-up to the clinical presentation of either “solitary plasmacytoma” (with minimal marrow involvement or less) or “smoldering (asymptomatic)” myeloma, again “corrected calcium” is listed as one of the recommended blood tests. Calcium is also recommended following treatment of active myeloma, and an elevated calcium concentration is listed as one of the “direct indicators of increasing disease and/or end organ dysfunction” since “excess bone resorption from bone disease can lead to excessive release of calcium into the blood, contributing to hypercalcemia.”
Occult Primary (Cancer of Unknown Primary [CUP]):48 “Routine laboratory tests (ie, complete blood count [CBC], electrolytes, liver function tests [LFTs], creatinine, calcium), occult blood stool testing, and contrast-enhanced chest/abdominal/pelvic CT scans with IV contrast are also recommended” for patients with a suspected metastatic malignancy.
Prostate Cancer:49 In the section concerning the treatment with denosumab, the guidelines state that “hypocalcemia is seen twice as often with denosumab than zoledronic acid and all patients on denosumab should be treated with vitamin D and calcium with periodic monitoring of serum calcium levels.” In the section concerning patients with castration resistant prostate cancer (CRPC), the NCCN states, “hypocalcemia should be corrected before starting denosumab, and serum calcium monitoring is required for denosumab and recommended for zoledronic acid, with repletion as needed.” In treatment of CRPC with abiraterone acetate, “monitoring of liver function, potassium and phosphate levels, and blood pressure readings on a monthly basis is warranted during abiraterone therapy.” Men with CRPC are at a higher risk for severe hypocalcemia and hypophosphatemia due to use of denosumab.
T-Cell Lymphomas:50 For adult T-Cell Leukemia/Lymphoma (ATLL), the NCCN states, “the initial workup for ATLL should include a complete history and physical examination…a CBC with differential and complete metabolic panel (serum electrolyte levels, calcium, creatinine, and blood urea nitrogen) and measurement of serum LDH levels.” Under the supportive care section for T-Cell lymphomas, the NCCN recommends monitoring for tumor lysis syndrome (TLS), which include measuring serum phosphorous and calcium levels since “laboratory TLS is defined as a 25% increase in the levels of serum uric acid, potassium, or phosphorus or a 25% decrease in calcium levels.”
Thyroid Carcinoma:51 In the algorithm for thyroid carcinoma-medullary carcinoma, both serum calcium and PTH are recommended as additional workup for patients who have MEN2A/Familial medullary thyroid carcinoma (codon 609, 611, 618, 620, 630, 634, 768, 790, 791, 804, or 891 RET mutations). Serum calcium testing is among the testing and procedures recommended upon diagnosis of medullary thyroid carcinoma.
2012, 2017, 2024 Kidney Disease Improving Global Outcomes (KDIGO)
Kidney Disease Improving Global Outcomes released their Clinical practice guideline for the Evaluation and Management of Chronic Kidney Disease (CKD) in 2012 and then their Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) in 2017. In the 2012 guidelines,52 in recommendation 3.3.1 (1C), they state, “We recommend measuring serum levels of calcium, phosphate, PTH, and alkaline phosphatase activity at least once in adults with GFR [glomerular filtration rate] <45 ml/min/1.73 m2 (GFR categories G3b-G5) in order to determine baseline values and inform prediction equations if used.” In recommendation 3.3.4 (2C recommendation strength), for people in GFR categories G3b-G5 they “suggest that people with levels of intact PTH above the upper normal limit of the assay are first evaluated for hyperphosphatemia, hypocalcemia, and vitamin D deficiency.” With regards to serum phosphate levels, they recommend that they are maintained “in the normal range according to local laboratory reference values” (recommendation 3.3.3; 2C). The guidelines, however, do not state a recommendation with respect to the frequency of testing past initial baseline and do not address magnesium testing other than to list renal magnesium wasting as a criterion for CKD.
The 2017 guidelines53 in recommendation 3.1.1 state: “We recommend monitoring serum levels of calcium, phosphate, PTH, and alkaline phosphatase activity beginning in CKD G3a (1C). In children, we suggest such monitoring beginning in CKD G2 (2D).” Recommendation 3.1.2 (Not graded) addresses the frequency of such testing and says, “to base the frequency…on the presence and magnitude of abnormalities, and the rate of progression of CKD.” The table below lists the “reasonable monitoring intervals”:
| CKD Stage |
Test |
Reasonable Monitoring Interval |
| G3a – G3b |
Serum Calcium |
Every 6 – 12 months |
| G3a – G3b |
Serum Phosphate |
Every 6 – 12 months |
| G3a – G3b |
PTH |
“Based on baseline level and CKD progression” |
| G4 |
Serum Calcium |
Every 3 – 6 months |
| G4 |
Serum Phosphate |
Every 3 – 6 months |
| G4 |
PTH |
Every 6 – 12 months |
| G5 |
Serum Calcium |
Every 1 – 3 months |
| G5 |
Serum Phosphate |
Every 1 – 3 months |
| G5 |
PTH |
Every 3 – 6 months |
| G4 – G5D |
Alkaline Phosphatase Activity |
Every 12 months, or more frequently in the presence of elevated PTH |
Recommendation 3.2.3 (2B) suggests measuring either PTH or bone-specific alkaline phosphatase to assess bone disease. For patients with CKD G3a-G5D, their treatment “should be based on serial assessments of phosphate, calcium, and PTH levels, considered together” (Recommendation 4.1.1 Not Graded). Recommendation 4.2.1 (2C) states: “In patients with CKD G3a-G5 not on dialysis, the optimal PTH level is not known. However, we suggest that patients with levels of intact PTH progressively rising or persistently above the upper normal limit for the assay be evaluated for modifiable factors, including hyperphosphatemia, hypocalcemia, high phosphate intake, and vitamin D deficiency.” Recommendation 5.2 (Not Graded) addressed the frequency of testing post-kidney transplant. The table below contains the information regarding the reasonable monitoring intervals:
| CKD Stage |
Test |
Reasonable Monitoring Interval |
| G1T – G3bT |
Serum Calcium |
Every 6 – 12 months |
| G1T – G3bT |
Serum Phosphate |
Every 6 – 12 months |
| G1T –G3bT |
PTH |
Once, with subsequent intervals depending on baseline level and CKD progression |
| G4T |
Serum Calcium |
Every 3 – 6 months |
| G4T |
Serum Phosphate |
Every 3 – 6 months |
| G4T |
PTH |
Every 6 – 12 months |
| G5T |
Serum Calcium |
Every 1 – 3 months |
| G5T |
Serum Phosphate |
Every 1 – 3 months |
| G5T |
PTH |
Every 3 – 6 months |
| G3aT – G5T |
Alkaline Phosphatase Activity |
Annually, or more frequently in the presence of elevated PTH |
Within recommendation 5.6 (2C), KDIGO recommends “treatment choices be influenced by the presence of CKD-MBD, as indicated by abnormal levels of calcium, phosphate, PTH, alkaline phosphatases, and 25(OH)D.”53
The 2024 guidelines54 in recommendation 1.3.1.1 state: “Use the following measurements for initial testing of albuminuria (in descending order of preference). In all cases, a first void in the morning midstream sample is preferred in adults and children.
If measuring urine protein, use the following measurements:
- urine ACR, or
- reagent strip urinalysis for albumin and ACR with automated reading.
If measuring urine protein, use the following measurements:
- urine protein-to-creatinine ratio (PCR),
- reagent strip urinalysis for total protein with automated reading, or
- reagent strip urinalysis for total protein with manual reading.
Recommendation 1.3.1.2 states: Use more accurate methods when albuminuria is detected using less accurate methods. Confirm reagent strip positive albuminuria and/or proteinuria by quantitative laboratory measurement and express as a ratio to urine creatinine wherever possible (i.e., quantify the ACR or PCR if initial semiquantitative tests are positive). Confirm ACR ‡30 mg/g (‡3 mg/mmol) on a random untimed urine with a subsequent first morning void in the morning midstream urine sample.
Practice Point 1.3.1.3: Understand factors that may affect interpretation of measurements of urine albumin and urine creatinine, and order confirmatory tests as indicated.
Recommendation 1.4.1 states: “We suggest that point-of-care testing (POCT) may be used for creatinine and urine albumin measurement where access to a laboratory is limited or providing a test at the point-of-care facilitates the clinical pathway (2C).
Practice Point 1.4.1: Whenever a POCT device is used for creatinine and urine albumin testing, ensure that the same preanalytical, analytical, and postanalytical quality criteria relating to the specimen collection and performance of the device, including external quality assessment, and the interpretation of the result is used.
Practice Point 1.4.2: Where a POCT device for creatinine testing is being used, generate an estimate of GFR. Use the equation consistent with that used within the region.
Practice Point 1.4.3: Where a POCT device is being used for albuminuria testing, the capability of also analyzing creatinine and producing an ACR is important. Assess the ability of the POCT ACR devices to produce a positive result in 85% of people with significant albuminuria (ACR ‡30 mg/g or ‡3 mg/ mmol), as part of the evaluation and consideration of using the device.”54
American Urological Association (AUA)
In 2013, the AUA published Follow-up for Clinically Localized Renal Neoplasms. In recommendation two, as an Expert Opinion, the AUA states, “Patients undergoing follow-up for treated or observed renal masses should undergo basic laboratory testing to include blood urea nitrogen (BUN)/creatinine, urine analysis (UA) and estimated glomerular filtration rate (eGFR). Other laboratory evaluations, including complete blood count (CBC), lactate dehydrogenase (LDH), liver function tests (LFTs), alkaline phosphatase (ALP) and calcium level, may be used at the discretion of the clinician.”
The AUA published their guidelines titled Medical Management of Kidney Stones in 2014. These guidelines were reviewed, and validity was confirmed in 2019.55 The purpose of the guideline was to provide a clinical framework for the diagnosis, prevention, and follow-up of adults with kidney stones. In recommendation two, the AUA recommends that “clinicians should obtain serum intact parathyroid hormone (PTH) level as part of the screening evaluation if primary hyperparathyroidism is suspected.” Also recommend (Recommendations six and seven) is that “metabolic testing should consist of one or two 24-hour urine collections obtained on a random diet and analyzed at minimum for total volume, pH, calcium, oxalate, uric acid, citrate, sodium, potassium and creatinine” but that “clinicians should not routinely perform ‘fast and calcium load’ testing to distinguish among types of hypercalciuria.”55
American Society for Bone and Mineral Research (ASBMR)
The ASBMR published a summary of recommendations for the diagnoses of chronic HypoPT. “Hypocalcemia (low ionized serum calcium or total serum calcium adjusted for albumin) in the presence of an undetectable, low or inappropriately normal intact PTH (utilizing either a second- or third-generation assay) on two occasions at least two weeks apart confirms the diagnosis. Additional abnormalities caused by low PTH that support the diagnosis: Elevation in serum phosphorus, reductions in 1,25-dihydroxyvitamin D (1,25(OH)2D) and elevations in the urinary fractional excretion of calcium. In individuals with postsurgical HypoPT, panel members regard the condition as permanent if the HypoPT persists more than 12 months after surgery.”6
The ASBMR completed a baseline laboratory profile that includes “calcium adjusted for albumin or ionized calcium, serum magnesium, phosphorus, creatinine and 25(OH)D, as well as 24-hour urine for creatinine and calcium. Stable individuals should be monitored with repeat laboratory profile every six to twelve months. Unstable individuals should be followed more closely to ensure that serum calcium does not fluctuate widely and to avoid the symptoms and the long-term complications of HypoPT. Ultrasound or computed tomography scan (CT) can be used during the evaluation of nephrocalcinosis or nephrolithiasis.”6
National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI)
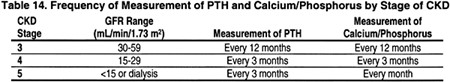
The NKF KDOQI published guidelines titled Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. Guideline 1.1 states that “Serum levels of calcium, phosphorus, and intact plasma parathyroid hormone (PTH) should be measured in all patients with CKD and GFR <60 mL/min/1.73 m2.”56 The table below contains a timeline of PTH and Calcium/Phosphorus measurements by CKD stage:
Recommendation 16.1 states that “serum levels of calcium, phosphorus, total CO2 and plasma intact PTH should be monitored following kidney transplantation. The frequency of these measurements should be based on the time following transplantation as shown in the table below:
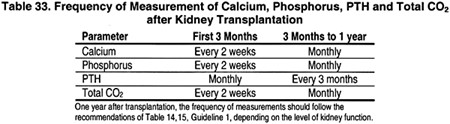
Recommendation 16.2 states that during the first week after kidney transplantation, serum levels of phosphorus should be measured daily. Kidney transplant recipients who develop persistently low levels of serum phosphate (<2.5 mg/dL [0.81 mmol/L]) should be treated with phosphate supplementation.”56
National Institute for Health and Care Excellence (NICE)
NICE, like the NCCN, addresses PTH, calcium, phosphate, and magnesium testing in several different guidelines.
2014 Bipolar disorder: assessment and management:57 In recommendation 1.2.12, they recommend annual calcium screening for anyone on a long-term lithium therapy regimen; however, in recommendation 1.10.21, they recommend testing “for urea and electrolytes including calcium…every six months, and more often if there is evidence of impaired renal or thyroid function, raised calcium levels or an increase in mood symptoms that might be related to impaired thyroid function.” In recommendation 1.10.14, when a patient begins a lithium regimen, a clinician should test “for urea and electrolytes including calcium, estimated glomerular filtration rate (eGFR), thyroid function and a full blood count.”
2014 Multiple sclerosis in adults: management:58 In recommendation 1.1.4, they recommend calcium testing along with full blood count, inflammatory markers, liver and renal function tests, glucose, thyroid function tests, vitamin B12, and HIV [human immunodeficiency virus] serology testing “before referring a person suspected of having MS to a neurologist” to “exclude alternative diagnoses.”
2015 Suspected cancer: recognition and referral:59 In the section concerning myeloma, in recommendation 1.10.4, they state, “offer a full blood count, blood tests for calcium and plasma viscosity or erythrocyte sedimentation rate to assess for myeloma in people aged 60 and over with persistent bone pain, particularly back pain, or unexplained fracture.”
2019 Clinical practice guideline: undernutrition in chronic kidney disease:60 These guidelines include a section regarding the nutritional status of an individual with chronic kidney disease. The NICE states that “Assessment of nutritional status should therefore be considered when patients begin education for kidney replacement treatment as part of their overall care as well as for potential intervention regarding salt, potassium, phosphate and protein / energy intake assessments.”60 Specific assessment methods are not mentioned.
2020 Hyperparathyroidism (primary) NICE guideline: diagnosis, assessment, and initial management:61 NICE states that “Initial diagnostic testing for suspected PHPT within primary care requires the measurement of albumin-adjusted serum calcium and PTH levels. Individuals should have their albumin-adjusted serum calcium measured if they have any of the following features: symptoms of hypercalcaemia such as thirst, frequent or excessive urination, or constipation; osteoporosis or previous fragility fracture; a renal stone; or an incidental finding of elevated albumin-adjusted serum calcium (≥2.6 mmol/L). The measurement of ionised calcium is not required when testing for suspected PHPT. The albumin-adjusted serum calcium measurement should be repeated in primary care at least once if the first measurement is either ≥2.6 mmol/L; or ≥2.5 mmol/L and features of PHPT are present. PTH should be measured in patients whose albumin-adjusted serum calcium level is either ≥2.6 mmol/L on at least two separate occasions; or ≥2.5 mmol/L on at least two separate occasions and PHPT is suspected. When measuring PTH, a random sample and a concurrent measurement of the albumin-adjusted
serum calcium are required. Repeat PTH measurements are not routinely required in primary care.”61
2021 Chronic kidney disease: assessment and management:62 In recommendation 1.11.9, within the section concerning the use of phosphate binders for children and young people, they state to “offer children and young people with CKD stage 4 or 5 and hyperphosphataemia a calcium-based phosphate binder to control serum phosphate levels.” In the continuation via recommendation 1.11.10, they also state, “if serum calcium increases towards, or above, the age-adjusted upper normal limit: ●investigate possible causes other than the phosphate binder ●consider reducing the dose of the calcium-based phosphate binder and adding sevelamer carbonate or switching to sevelamer carbonate alone [2021].” When discussing phosphate binders for adults, they state in their recommendation 1.11.12 for the first phosphate binder, “offer adults with CKD stage 4 or 5 and hyperphosphataemia calcium acetate to control serum phosphate levels [2021].” If calcium acetate is not indicated, “for example, because of hypercalcaemia or low serum parathyroid hormone levels,” or not tolerated, recommendation 1.11.13 states to offer sevelamer carbonate. Recommendations 1.11.14 and 1.11.15 continue by offering sucroferric oxyhydroxide, if an adult is on dialysis and a calcium-based phosphate binder is not needed; calcium carbonate, “if a calcium-based phosphate binder is needed”; and lanthanum carbonate “for adults with CKD stage 4 or 5 if other phosphate binders cannot be used.” In the 2021 update, they also state in recommendation 1.11.18, “at every routine clinical review, assess the person’s serum phosphate control, taking into account: ●diet ●whether they are taking the phosphate binders as prescribed ●other relevant factors, such as vitamin D levels, serum parathyroid hormone levels, alkaline phosphatase, serum calcium, medications that might affect serum phosphate, or dialysis [2021].” These guidelines mention serum phosphate, serum calcium, and PTH; however, they do not state when these tests should be performed or the frequency of testing.
In recommendation 1.12.1, they do not recommend to “routinely measure calcium, phosphate, parathyroid hormone (PTH) and vitamin D levels in people with a GFR of 30 ml/min/1.73 m2 or more (GFR category G1, G2, or G3).” Then, in the following recommendation, they do recommend measuring serum calcium, PTH, and phosphate for patients in GFR categories G4 or G5. “Determine the subsequent frequency of testing by the measured values and the clinical circumstances. If doubt exists, seek specialist opinion.” They recommend in 1.12.7 to “monitor serum calcium and phosphate concentrations in people receiving alfacalcidol or calcitriol supplements.”
European Society of Endocrinology
The ESE summarized the hypoparathyroidism (HypoPT) consensus recommendations published by the “European Expert Consensus on Practical Management of Specific Aspects of Parathyroid Disorders in Adults and in Pregnancy.” In this summary, the ESE state that Calcium testing looking for hypocalcemia and hypercalcemia should occur at “every 3 to 6 months or every check at steady state. Ionized calcium is preferable. If not available, total calcium (and albumin-corrected) is acceptable timing of assessment is dependent on previous/daily calcium intake by food or supplements, as well as treatment. Calcium levels should be assessed several days after changes in active vitamin D analog doses or PTH doses to detect iatrogenic hypercalcemia. PTH testing should only occur for diagnosis. It is not required for follow-up in chronic HypoPT. It should be assessed to detect recovery in transient postsurgical hypoparathyroidism (6-12 months after surgery). Phosphate Hyperphosphatemia should occur every three to six months or at every check. Hyperphosphatemia can be related to high dietary phosphate intake (soft drinks, products with preservatives, acidifier, and flavor enhancer). Hyperphosphatemia is associated with higher risk of infections and with increased mortality.”63
2021 American Society of Clinical Oncology (ASCO)/Cancer Care Ontario
The CCO and ASCO convened a working group in 2017 concerning the use of bisphosphonates in breast cancer and published their recommendations in the Journal of Clinical Oncology. They clearly state that “patients should have serum calcium measured prior to starting treatment. Patients receiving intravenous bisphosphonates (zoledronic acid) should be monitored for renal function prior to starting this treatment, and for serum calcium and increase in serum creatinine throughout the treatment period.”
A 2021 update from the CCO and ASCO group reaffirmed the statement above.64
American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE)
In 2020, the AACE/ACE updated its 2016 guidelines concerning osteoporosis in post-menopausal women. To assess for causes of secondary osteoporosis for all women with osteoporosis, the AACE/ACE recommends the following laboratory tests: “a complete blood count, comprehensive metabolic panel, 25-hydroxyvitamin D (25[OH]D), intact parathyroid hormone (PTH), phosphate, and a 24-hour urine collection for calcium, sodium, and creatinine” in evaluating osteoporosis. The guidelines note that “the 24-hour urine calcium collection must occur after the patient is replete of vitamin D and has been on a reasonable calcium intake (1,000-1,200 mg/day) for at least 2 weeks. If the patient is receiving thyroid hormone or there is suspicion for hyperthyroidism, thyroid-stimulating hormone should also be obtained.”65
In the 2017 guidelines for the management of dyslipidemia prevention of cardiovascular disease, the AACE/ACE highlighted the use of coronary artery calcium scores in the detection of cardiovascular risk, stating that coronary artery calcium scoring “is recognized by the AHA [American Heart Association] as a surrogate marker for coronary heart disease.”66
2014 and 2022 Society of Obstetricians and Gynaecologists of Canada (SOGC)
The 2014 SOGC guidelines concerning hypertensive disorders during pregnancy recommend using magnesium supplements for pregnant women; however, the SOGC clearly states in recommendation #120 that “routine monitoring of serum magnesium levels is not recommended.”67
However, in the updated 2022 guidelines there is no mention of magnesium testing, only a recommendation for magnesium sulphate as a first-line treatment of eclampsia and prophylaxis against eclampsia in women with preeclampsia and severe hypertension or adverse maternal conditions (strong, high).68
2022 American Heart Association /American College of Cardiology /Heart Failure Society of America (HSFA) Guideline for the Management of Heart Failure
The 2022 guideline concerning heart failure mentions both magnesium and calcium testing for patients with heart failure (HF), “Laboratory evaluation with complete blood count, urinalysis, serum electrolytes (including sodium, potassium, calcium, and magnesium), blood urea nitrogen, serum creatinine, glucose, fasting lipid profile, liver function tests, iron studies (serum iron, ferritin, transferrin saturation), and thyroid-stimulating hormone level and electrocardiography is part of the standard diagnostic evaluation of a patient with HF.”69
2016 American Academy of Pediatrics (AAP)
The AAP in 2016 issued guidelines concerning brief resolved unexplained events (BRUE) in infants. “The term BRUE is defined as an event occurring in an infant younger than 1 year when the observer reports a sudden, brief, and now resolved episode of ≥1 of the following: (1) cyanosis or pallor;70 absent, decreased, or irregular breathing;71 marked change in tone (hyper- or hypotonia); and (4) altered level of responsiveness.” For infants between 60 days and <1 year in age, in recommendation 6B under IEM (inborn error of metabolism), the AAP states that “clinicians should not obtain a measurement of serum sodium, potassium, chloride, blood urea nitrogen, creatinine, calcium, or ammonia to detect an IEM on infants presenting with a lower-risk BRUE (Grade C, Moderate Recommendation).”70
2013 American Association of Clinical Endocrinologists (AACE)/American College of Endocrinology (ACE)/The Obesity Society (TOS)
The joint task force between AACE, ACE, and TOS issued Clinical Practice Guidelines for Healthy Eating for the Prevention and Treatment of Metabolic and Endocrine Diseases in Adults in 2013. With regards to CKD in recommendation R29, they state, “If the intact parathyroid hormone (PTH) level remains elevated above treatment goal despite a serum 25(OH)D level higher than 30 ng/mL, treatment with an active form of vitamin D is indicated (Grade A, BEL1).” As part of recommendation R32, they state, “A 24-hour urine calcium collection should be measured in patients with osteoporosis or patients at risk for bone loss in order to check calcium adequacy and test for hypercalciuria or malabsorption (Grade B, BEL 2).” Furthermore, “during vitamin D therapy, serum calcium and phosphorus levels need to be monitored closely to prevent hypercalcemia and hyperphosphatemia, aiming for calcium and phosphorus levels of <10.2 mg/dL and <4.6 mg/dL, respectively.”72
2013, 2019 AACE/TOS/ASMBS (American Society for Metabolic & Bariatric Surgery)/OMA (Obesity Medical Association)/ASA (American Society of Anesthesiologists)
Also, in 2013, the AACE/TOS/ASMBS/OMA/ASA issued guidelines concerning perioperative, nonsurgical support for the bariatric surgery patient. Within recommendation R48, they state, “Bisphosphonates may be considered in bariatric surgery patients with osteoporosis only after appropriate therapy for calcium and vitamin D insufficiency…. Evaluation should include serum parathyroid hormone (PTH), total calcium, phosphorus, 25-hydroxyvitamin D, and 24-hour urine calcium levels (Grade C; BEL 3).”
The updated guidelines for the perioperative nutrition, metabolic, and nonsurgical support of patients undergoing bariatric procedures were published by the ACE, TOS, ASMBS as well as the Obesity Medicine Association, and the American Society of Anesthesiologists Boards of Directors. The guidelines give the following recommendations:
- “Patients who become pregnant following bariatric procedure should have nutritional surveillance and laboratory screening for nutrient deficiencies every trimester, including iron, folate, vitamin B12, vitamin D, and calcium, and if after a malabsorptive procedure, fat-soluble vitamins, zinc, and copper (Grade D)
- Evaluation of patients for bone loss after bariatric procedures may include serum parathyroid hormone, total calcium, phosphorus, 25-hydroxyvitamin D, and 24-hour urine calcium levels (Grade C; BEL 3).”73
2013 American Gastroenterological Association (AGA)
The 2013 AGA guidelines concerning constipation states that “although metabolic tests (thyroid-stimulating hormone, serum glucose, creatinine, and calcium) are often performed, their diagnostic utility and cost-effectiveness have not been rigorously evaluated and are probably low.” Under the section What Tests Should Be Performed to Assess for Medical Causes of Constipation?, they state, “In the absence of other symptoms and signs, only a complete blood cell count is necessary (strong recommendation, low-quality evidence). Unless other clinical features warrant otherwise, metabolic tests (glucose, calcium, sensitive thyroid-stimulating hormone) are not recommended for chronic constipation (strong recommendation, moderate-quality evidence).”74
American Thyroid Association (ATA)
The ATA has published guidelines for the diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. The ATA has stated that after a thyroidectomy, “serum calcium with or without intact parathyroid hormone (iPTH) levels can be measured,”; further, after a thyroidectomy for TMNG (toxic multinodular goiter), “serum calcium with or without iPTH
levels should be measured.”75 When preparing patients with GD (Graves' disease) for a thyroidectomy, the ATA recommends that “Calcium and 25-hydroxy vitamin D should be assessed preoperatively and repleted if necessary.”75
The ATA also published a statement regarding postoperative hypoparathyroidism. In it, they recommend to “Either treat at-risk patients empirically with calcium, or measure calcium and/or PTH in the immediate postoperative period and treat according to evidence-based protocols.”71
References
- Mannstadt M. Parathyroid hormone secretion and action. Updated February 25, 2025. https://www.uptodate.com/contents/parathyroid-hormone-secretion-and-action
- Goyal A, Anastasopoulou C, Ngu M, Singh S. Hypocalcemia. 2023. https://www.ncbi.nlm.nih.gov/books/NBK430912/
- Fuleihan GE-H, Silverberg SJ. Primary hyperparathyroidism: Diagnosis, differential diagnosis, and evaluation. Updated September 28, 2023. https://www.uptodate.com/contents/primary-hyperparathyroidism-diagnosis-differential-diagnosis-and-evaluation
- Stubbs JR, Yu ASL. Hypophosphatemia: Evaluation and treatment. Updated March 6, 2024. https://www.uptodate.com/contents/hypophosphatemia-evaluation-and-treatment
- Workinger JL, Doyle RP, Bortz J. Challenges in the Diagnosis of Magnesium Status. Nutrients. 2018;10(9):1202. doi:10.3390/nu10091202
- Khan AA, Bilezikian JP, Brandi ML, et al. Evaluation and Management of Hypoparathyroidism Summary Statement and Guidelines from the Second International Workshop. J Bone Miner Res. Dec 2022;37(12):2568-2585. doi:10.1002/jbmr.4691
- Mantovani G, Bastepe M, Monk D, et al. Diagnosis and management of pseudohypoparathyroidism and related disorders: first international Consensus Statement. Nature reviews Endocrinology. Jun 29 2018;doi:10.1038/s41574-018-0042-0
- Valcour A, Zierold C, Blocki FA, et al. Trueness, precision and stability of the LIAISON 1-84 parathyroid hormone (PTH) third-generation assay: comparison to existing intact PTH assays. Clinical chemistry and laboratory medicine. May 11 2018;doi:10.1515/cclm-2018-0217
- Fuleihan GE-H, Juppner H. Parathyroid hormone assays and their clinical use. Updated March 22, 2024. https://www.uptodate.com/contents/parathyroid-hormone-assays-and-their-clinical-use
- Wojda SJ, Donahue SW. Parathyroid hormone for bone regeneration. J Orthop Res. Oct 2018;36(10):2586-2594. doi:10.1002/jor.24075
- Leder BZ. Parathyroid Hormone and Parathyroid Hormone-Related Protein Analogs in Osteoporosis Therapy. Curr Osteoporos Rep. Apr 2017;15(2):110-119. doi:10.1007/s11914-017-0353-4
- Bislev LS, Langagergaard Rodbro L, Sikjaer T, Rejnmark L. Effects of Elevated Parathyroid Hormone Levels on Muscle Health, Postural Stability and Quality of Life in Vitamin D-Insufficient Healthy Women: A Cross-Sectional Study. Calcif Tissue Int. Dec 2019;105(6):642-650. doi:10.1007/s00223-019-00612-2
- Marcucci G, Della Pepa G, Brandi ML. Drug safety evaluation of parathyroid hormone for hypocalcemia in patients with hypoparathyroidism. Expert Opin Drug Saf. May 2017;16(5):617-625. doi:10.1080/14740338.2017.1311322
- Rodriguez-Ortiz ME, Canalejo A, Herencia C, et al. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentration. Nephrol Dial Transplant. Feb 2014;29(2):282-9. doi:10.1093/ndt/gft400
- Shaker JL, Deftos L. Calcium and Phosphate Homeostasis. In: Feingold KR, Anawalt B, Boyce A, et al, eds. Endotext. MDText.com, Inc.; 2023.
- Han CH, Fry CH, Sharma P, Han TS. A clinical perspective of parathyroid hormone related hypercalcaemia. Rev Endocr Metab Disord. Dec 3 2019;doi:10.1007/s11154-019-09529-5
- Lederer E. Regulation of serum phosphate. The Journal of physiology. Sep 15 2014;592(18):3985-95. doi:10.1113/jphysiol.2014.273979
- Quamme GA. Renal handling of magnesium: drug and hormone interactions. Magnesium. 1986;5(5-6):248-72.
- Konishi H, Fujiwara H, Itoh H, et al. Influence of magnesium and parathyroid hormone on cisplatin-induced nephrotoxicity in esophageal squamous cell carcinoma. Oncology letters. Jan 2018;15(1):658-664. doi:10.3892/ol.2017.7345
- Hernandez-Becerra E, Jimenez-Mendoza D, Mutis-Gonzalez N, Pineda-Gomez P, Rojas-Molina I, Rodriguez-Garcia ME. Calcium Deficiency in Diet Decreases the Magnesium Content in Bone and Affects Femur Physicochemical Properties in Growing Rats. Biol Trace Elem Res. Jan 9 2020;doi:10.1007/s12011-019-01989-9
- Hanon EA, Sturgeon CM, Lamb EJ. Sampling and storage conditions influencing the measurement of parathyroid hormone in blood samples: a systematic review. Clinical chemistry and laboratory medicine. Oct 2013;51(10):1925-41. doi:10.1515/cclm-2013-0315
- Sturgeon CM, Sprague S, Almond A, et al. Perspective and priorities for improvement of parathyroid hormone (PTH) measurement - A view from the IFCC Working Group for PTH. Clinica chimica acta; international journal of clinical chemistry. Apr 2017;467:42-47. doi:10.1016/j.cca.2016.10.016
- Almond A, Ellis AR, Walker SW. Current parathyroid hormone immunoassays do not adequately meet the needs of patients with chronic kidney disease. Annals of clinical biochemistry. Jan 2012;49(Pt 1):63-7. doi:10.1258/acb.2011.011094
- Bensalah M, Bouayadi O, Rahmani N, Lyagoubi A, Lamrabat S, Choukri M. Comparative study of the serum measurement of PTH on Roche Cobas e411((R)) versus the Abbott Architect ci8200((R)). Ann Biol Clin (Paris). Jan 1 2018;76(1):61-67. Etude comparative du dosage de la PTH serique sur Architect ci8200((R)) versus Cobas e411((R)). doi:10.1684/abc.2017.1309
- Press DM, Siperstein AE, Berber E, et al. The prevalence of undiagnosed and unrecognized primary hyperparathyroidism: a population-based analysis from the electronic medical record. Surgery. Dec 2013;154(6):1232-7; discussion 1237-8. doi:10.1016/j.surg.2013.06.051
- Pak CYC, Kaplan R, Bone H, Townsend J, Waters O. A Simple Test for the Diagnosis of Absorptive, Resorptive and Renal Hypercalciurias. New England Journal of Medicine. 1975/03/06 1975;292(10):497-500. doi:10.1056/NEJM197503062921002
- Mayo. Calcium, 24 Hour, Urine. https://www.mayocliniclabs.com/test-catalog/overview/610595#Clinical-and-Interpretive
- Curhan GC, Willett WC, Speizer FE, Stampfer MJ. Twenty-four-hour urine chemistries and the risk of kidney stones among women and men. Kidney international. Jun 2001;59(6):2290-8. doi:10.1046/j.1523-1755.2001.00746.x
- Mayo. Magnesium, Serum. https://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8448
- Kreepala C, Kitporntheranunt M, Sangwipasnapaporn W, Rungsrithananon W, Wattanavaekin K. Assessment of preeclampsia risk by use of serum ionized magnesium-based equation. Renal failure. Nov 2018;40(1):99-106. doi:10.1080/0886022x.2017.1422518
- Hamedanian L, Badehnoosh B, Razavi-Khorasani N, Mohammadpour Z, Mozaffari-Khosravi H. Evaluation of vitamin D status, parathyroid hormone, and calcium among Iranian pregnant women with preeclampsia: A case-control study. Int J Reprod Biomed (Yazd). Dec 2019;17(11):831-840. doi:10.18502/ijrm.v17i10.5494
- Rooney MR, Alonso A, Folsom AR, et al. Serum magnesium and the incidence of coronary artery disease over a median 27 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study and a meta-analysis. Am J Clin Nutr. Jan 1 2020;111(1):52-60. doi:10.1093/ajcn/nqz256
- Mancuso E, Perticone M, Spiga R, et al. Association between Serum Mg(2+) Concentrations and Cardiovascular Organ Damage in a Cohort of Adult Subjects. Nutrients. 2020;12(5):1264. doi:10.3390/nu12051264
- Sri-Ganeshan M, Walker KP, Lines TJ, et al. Evaluation of a calcium, magnesium and phosphate clinical ordering tool in the emergency department. Am J Emerg Med. Mar 2022;53:163-167. doi:10.1016/j.ajem.2022.01.003
- Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons Guidelines for Definitive Management of Primary Hyperparathyroidism. JAMA Surgery. 2016;151(10):959-968. doi:10.1001/jamasurg.2016.2310
- Mantovani G, Bastepe M, Monk D, et al. Recommendations for Diagnosis and Treatment of Pseudohypoparathyroidism and Related Disorders: An Updated Practical Tool for Physicians and Patients. Hormone Research in Paediatrics. 2020;93(3):182-196. doi:10.1159/000508985
- Bilezikian JP, Khan AA, Clarke BL, Mannstadt M, Potts JT, Brandi ML. The Fifth International Workshop on the Evaluation and Management of Primary Hyperparathyroidism. Journal of Bone and Mineral Research. 2022;37(11):2290-2292. doi:10.1002/jbmr.4670
- Bilezikian JP, Brandi ML, Eastell R, et al. Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the Fourth International Workshop. J Clin Endocrinol Metab. Oct 2014;99(10):3561-9. doi:10.1210/jc.2014-1413
- NCCN. Neuroendocrine and Adrenal Tumors. Updated January 17, 2025. https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- NCCN. Acute Lymphoblastic Leukemia Version 3.2024. Updated December 20,2024. https://www.nccn.org/professionals/physician_gls/pdf/all.pdf
- NCCN. Systemic Light Chain Amyloidosis - Version 1.2025. Accessed 12/12/23, https://www.nccn.org/professionals/physician_gls/pdf/amyloidosis.pdf
- NCCN. Bone Cancer - Version 1.2025. Updated August 20, 2024. https://www.nccn.org/professionals/physician_gls/pdf/bone.pdf
- NCCN. Breast Cancer - Version 1.2025. Updated January 31, 2025. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- NCCN. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma - Version 1.2025. Updated October 1, 2024. https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf
- NCCN. Esophageal and Esphagogastric Junction Cancers - Version 5.2024. Updated December 20, 2024. https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- NCCN. Kidney Cancer - Version 3.2025. Updated January 09, 2025. https://www.nccn.org/professionals/physician_gls/pdf/kidney.pdf
- NCCN. Multiple Myeloma Version 1. 2025. Updated September 17,2024. https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf
- NCCN. Occult Primary (Cancer of Unknown Primary [CUP]) - Version 2.2025. Updated September 11, 2024. https://www.nccn.org/professionals/physician_gls/pdf/occult.pdf
- NCCN. Prostate Cancer - Version 1.2025. Updated December 4, 2024. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- NCCN. T-Cell Lymphomas - Version 1.2025. Updated November 11,2024. https://www.nccn.org/professionals/physician_gls/pdf/t-cell.pdf
- NCCN. Thyroid Carcinoma - Version 5.2024. Updated January 15,2025. https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf
- KDIGO. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney international. 2013;3(1):v - 150.
- KDIGO. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD). Kidney international. July 2017 2017;7(1):1-59.
- KDIGO. KDIGO 2024 CLINICAL PRACTICE GUIDELINE FOR THE EVALUATION AND MANAGEMENT OF CHRONIC KIDNEY DISEASE. 2024;105(4S)
- Pearle MS, Goldfarb DS, Assimos DG, et al. Medical Management of Kidney Stones: AUA Guideline. Journal of Urology. 2019;
- KDOQI. KDOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease. NKF KDOQI. 2003
- NCCMH. National Institute for Health and Care Excellence: Clinical Guidelines. Bipolar Disorder: The NICE Guideline on the Assessment and Management of Bipolar Disorder in Adults, Children and Young People in Primary and Secondary Care. British Psychological Society (c) The British Psychological Society & The Royal College of Psychiatrists, 2014.; 2023.
- NICE. Multiple sclerosis in adults: management. Updated June 22, 2022. https://www.nice.org.uk/guidance/ng220
- NICE. National Institute for Health and Care Excellence: Clinical Guidelines. Suspected Cancer: Recognition and Referral. National Institute for Health and Care Excellence (UK) Copyright (c) National Collaborating Centre for Cancer.; 2023.
- Wright M, Southcott E, MacLaughlin H, Wineberg S. Clinical practice guideline on undernutrition in chronic kidney disease. BMC Nephrol. Oct 16 2019;20(1):370. doi:10.1186/s12882-019-1530-8
- Jawaid I, Rajesh S. Hyperparathyroidism (primary) NICE guideline: diagnosis, assessment, and initial management. 2020;
- NICE. Chronic kidney disease: assessment and management. Updated August 25, 2021. https://www.nice.org.uk/guidance/ng203
- Endocrinology ESo. Managing Parathyroid Disorders: Chronic Hypoparathyroidism. European Journal of Endocrinology 2022;2
- Eisen A, Somerfield MR, Accordino MK, et al. Use of Adjuvant Bisphosphonates and Other Bone-Modifying Agents in Breast Cancer: ASCO-OH (CCO) Guideline Update. Journal of Clinical Oncology. 2022;40(7):787-800. doi:10.1200/jco.21.02647
- Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2020 Update. Endocrine Practice. 2020/05/01/ 2020;26:1-46. doi:10.4158/GL-2020-0524SUPPL
- Jellinger PS, Handelsman Y, Rosenblit PD, et al. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY GUIDELINES FOR MANAGEMENT OF DYSLIPIDEMIA AND PREVENTION OF CARDIOVASCULAR DISEASE. Endocrine Practice. 2017;23(2)
- Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. May 2014;36(5):416-41.
- Magee LA, Smith GN, Bloch C, et al. Guideline No. 426: Hypertensive Disorders of Pregnancy: Diagnosis, Prediction, Prevention, and Management. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. May 2022;44(5):547-571.e1. doi:10.1016/j.jogc.2022.03.002
- Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895-e1032. doi:doi:10.1161/CIR.0000000000001063
- Tieder JS, Bonkowsky JL, Etzel RA, et al. Brief Resolved Unexplained Events (Formerly Apparent Life-Threatening Events) and Evaluation of Lower-Risk Infants. Pediatrics. May 2016;137(5)doi:10.1542/peds.2016-0590
- Orloff LA, Wiseman SM, Bernet VJ, et al. American Thyroid Association Statement on Postoperative Hypoparathyroidism: Diagnosis, Prevention, and Management in Adults. Thyroid. Jul 2018;28(7):830-841. doi:10.1089/thy.2017.0309
- Gonzalez-Campoy JM, St Jeor ST, Castorino K, et al. Clinical practice guidelines for healthy eating for the prevention and treatment of metabolic and endocrine diseases in adults: cosponsored by the American Association of Clinical Endocrinologists/the American College of Endocrinology and the Obesity Society. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. Sep-Oct 2013;19 Suppl 3:1-82. doi:10.4158/ep13155.Gl
- Mechanick JI, Apovian C, Brethauer S, et al. Clinical Practice Guidelines for The Perioperative Nutrition, Metabolic, And Nonsurgical Support of Patients Undergoing Bariatric Procedures - 2019 Update: Cosponsored by American Association of Clinical Endocrinologists/American College of Endocrinology, The Obesity Society, American Society for Metabolic & Bariatric Surgery, Obesity Medicine Association, and American Society of Anesthesiologists - Executive Summary. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. Dec 2019;25(12):1346-1359. doi:10.4158/gl-2019-0406
- Association AG. American Gastroenterological Association Medical Position Statement on Constipation. AGA 2013;144(1)doi: 10.1053/j.gastro.2012.10.029
- Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. Oct 2016;26(10):1343-1421. doi:10.1089/thy.2016.0229
Coding Section
| Code |
Number |
Description |
| CPT |
82310 |
Calcium; total |
|
|
82330 |
Calcium; ionized |
|
|
82340 |
Calcium; urine quantitative, timed specimen |
|
|
83735 |
Magnesium |
|
|
83970 |
Parathormone (parathyroid hormone) |
|
|
84100 |
Phosphorus inorganic (phosphate) |
|
|
84105 |
Phosphorus inorganic (phosphate); urine |
| ICD-10 CM |
E83.52 |
Hypercalcemia |
| Q87.11 (Effective 10/01/2019) |
Prader-Willi syndrome |
|
|
|
Q87.19 (Effective 10/01/2019) |
Other congenital malformation syndromes predominantly associated with short stature |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2018 Forward
| 05/06/2025 | Annual review, updating coverage criteria 1 to combine criteria 1 and 2. Also updating description, table of terminology, rationale, guidelines/recommendations, and references. |
| 04/26/2024 | Annual review, no change to policy intent. Updating rationale, references, regulatory status. |
| 04/19/2023 | Annual review, no change to policy intent, but, policy is rewritten for clarity and consistency. Also updating description, rationale and references. |
| 04/13/2022 |
Annual review, adding "in patients with hypercalcemia" to medical necessity statement #1. Also updating rationale and references. Adding table of terminology. |
| 04/01/2021 |
Annual review, no change to policy intent. Updating rationale and references. |
| 04/14/2020 |
Annual review , no change to policy intent. |
| 09/30/2019 |
Added ICD-10 codes to Coding Section. No other changes Made. |
| 04/01/2019 |
New Policy |