Testosterone - CAM 131
Description/Background
Testosterone is a naturally occurring lipophilic androgen hormone that is produced by both males and females for various functions. In males, testosterone is produced by the interstitial cells of Leydig in the testis. In females, testosterone is primarily created and disseminated by the ovaries and adrenal glands. Testosterone is required for synthesis of dihydrotestosterone (DHT) as well as estradiol (E2). Sex hormone-binding globulin (SHBG) binds testosterone to aid in transport and intratesticular bioavailability.
Dysregulation in testosterone levels can lead to serious conditions, including hypogonadism and other testosterone excess or deficiency conditions. Additional hormones, including follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin, play roles in development. As part of the hypothalamic-pituitary-gonadal axis, FSH and LH bind to gonadal receptors to modulate testosterone. During conditions of dyshomeostasis, such as hypogonadism, FSH, LH, and prolactin serum levels can be used as diagnostic tools (Bhasin et al., 2018; Gill-Sharma, 2018).
Terms such as male and female are used when necessary to refer to sex assigned at birth.
Policy
- Measurement of serum total testosterone (see Note 1) is considered MEDICALLY NECESSARY in any of the following situations:
- For symptoms of androgen deficiency or androgen excess in males:
- For initial screening, two measurements at least 24 hours apart.
- If the initial screening was normal but symptoms persist, follow-up testing is allowed no sooner than 60 days after the initial screening.
- For the monitoring of treatment response in men taking enzyme inhibitors for prostate cancer.
- For men receiving testosterone replacement therapy (every 2 – 3 months for the first year after initiation of therapy or after a change in therapeutic dosage; annually thereafter).
- For gender-dysphoric/gender-incongruent persons (baseline, during treatment, and for therapy monitoring).
- For symptomatic females (see Note 2) being evaluated for conditions associated with androgen excess (e.g., polycystic ovary syndrome and functional hypothalamic amenorrhea).
- For symptoms of androgen deficiency or androgen excess in males:
- For males with total testosterone confirmed as low or borderline low and who have hypogonadism, gynecomastia, and/or other forms of testicular hypofunction, annual measurement of serum free testosterone, sex hormone-binding globulin (SHBG), and/or albumin is considered MEDICALLY NECESSARY.
- For individuals suspected of having a disorder that is accompanied by increased or decreased SHBG levels (see Notes 3 and 4), measurement of serum free testosterone using a medically accepted algorithm based on total serum testosterone, SHBG, and/or albumin or bioavailable testosterone is considered MEDICALLY NECESSARY.
- Prior to initiating testosterone therapy for males with gynecomastia, once per lifetime serum estradiol measurement is considered MEDICALLY NECESSARY.
- For individuals with ambiguous genitalia, hypospadias, or microphallus, measurement of serum dihydrotestosterone for the diagnosis of 5-alpha reductase deficiency is considered MEDICALLY NECESSARY.
- Measurement of serum free testosterone and/or bioavailable testosterone as a primary test (i.e., in the absence of prior serum total testosterone measurement) is considered NOT MEDICALLY NECESSARY.
- For asymptomatic individuals or for individuals with non-specific symptoms, measurement of serum total testosterone, free testosterone, and/or bioavailable testosterone is considered NOT MEDICALLY NECESSARY.
- For the identification of androgen deficiency in women, measurement of serum testosterone is considered NOT MEDICALLY NECESSARY.
The following does not meet coverage criteria due to a lack of available published scientific literature confirming that the test(s) is/are required and beneficial for the diagnosis and treatment of a patient’s illness.
- The use of saliva for the measurement of testosterone is considered NOT MEDICALLY NECESSARY.
- For all other situations not mentioned above, measurement of serum dihydrotestosterone is considered NOT MEDICALLY NECESSARY.NOTES:
Note 1: Serum total testosterone sample collection should occur in the early morning, after fasting. Due to considerable variability in serum total testosterone levels, the Centers for Disease Control and Prevention (CDC) developed a standardization program for total testosterone assays (Hormone Standardization [HoSt]/Testosterone). An assay certified by the CDC’s HoSt/Testosterone program is standardized to within ±6.4% of the CDC total testosterone reference standard. It is STRONGLY RECOMMENDED that serum total testosterone measurement be performed with an assay that has been certified by the CDC HoSt/Testosterone program (Bhasin et al., 2018). A list of CDC-certified assays is available on the HoSt website (CDC, 2023).
Note 2: When measuring serum total testosterone in females, please note that the technology used for measurement must be sensitive enough to detect the low serum total testosterone levels that are normally found in females.
Note 3: Conditions associated with decreased SHBG concentrations according to the 2018 Endocrine Society Guidelines (Bhasin et al., 2018):
- Obesity
- Diabetes mellitus
- Use of glucocorticoids, progestins, and androgenic steroids
- Nephrotic syndrome
- Hypothyroidism
- Acromegaly
- Polymorphisms in the SHBG gene
Note 4: Conditions associated with increased SHBG concentrations according to the 2018 Endocrine Society Guidelines (Bhasin et al., 2018):
- Aging
- HIV disease
- Cirrhosis and hepatitis
- Hyperthyroidism
- Use of some anticonvulsants
- Use of estrogens
- Polymorphisms in the SHBG gene
Table of Terminology
| Term |
Definition |
| AAP |
American Academy of Pediatrics |
| ABIM |
American Board of Internal Medicine |
| ABP |
Androgen binding protein |
| ACOG |
The American College of Obstetricians and Gynecologists |
| ACTH |
Adrenocorticotropic hormone |
| ADT |
Androgen deprivation therapy |
| AFP |
Alpha- fetal protein |
| ASRM |
American Society of Reproductive Medicine |
| AIDS |
Acquired immunodeficiency syndrome |
| AUA |
American Urological Association |
| CBG |
Corticosteroid-binding globulin-bound testosterone |
| CDC |
Centers for Disease Control and Prevention |
| CLIA’88 |
Clinical Laboratory Improvement Amendments of 1988 |
| CMAJ |
Canadian Medical Association Journal |
| CMS |
Centers for Medicare & Medicaid Services |
| CSAM |
Canadian Society of Endocrinology and Metabolism |
| CUA |
Canadian Urological Association |
| CV |
Coefficient of variation |
| DHEAS |
Dehydroepianandrosterone sulphate |
| DHT |
Dihydrotestosterone |
| E2 |
Estradiol |
| EAA |
European Academy of Andrology |
| EAU |
European Association of Urology |
| ELISA |
Enzyme linked immunosorbent assay |
| ES |
The Endocrine Society |
| ESI |
Electrospray ionization |
| FDA |
Food and Drug Administration |
| FHA |
Functional hypothalamic amenorrhea |
| FSH |
Follicle-stimulating hormone |
| FT |
Free testosterone |
| Gy |
Gray unit of ionizing radiation |
| hCG |
Human chorionic gonadotropin |
| HIV |
Human immunodeficiency virus |
| HoSt |
Hormone standardization |
| HPLC |
High performance liquid chromatography |
| ID |
Isotope dilution |
| ID-LC-MS |
Isotope dilution-liquid chromatography-tandem mass spectrometry |
| IGHG |
International Late Effects of Childhood Cancer Guideline Harmonization Group |
| LC-MS/MS |
Liquid chromatography-tandem mass spectrometry |
| LDTs |
Laboratory-developed tests |
| LH |
Luteinizing hormone |
| LOH |
Late onset hypogonadism |
| MRI |
Magnetic resonance imaging |
| MS |
Mass spectrometry |
| NCCN |
National Comprehensive Cancer Network |
| OH |
Hydroxy |
| ORM |
Orosomucoid bound testosterone |
| PADAM |
Partial androgen deficiency |
| PCOS |
Polycystic ovary syndrome |
| PCSF |
PanCareSurFup |
| PRL |
Prolactin |
| PSA |
Prostatic specific antigen |
| SHBG |
Sex hormone binding globulin |
| SHBG |
Sex hormone binding globulin-bound testosterone |
| TBI |
Traumatic Brain Injury |
| TBS |
Trabecular bone score |
| THS |
Tetrahydrocortisol |
| TSH |
Thyroid stimulating hormone |
| TT |
Total testosterone |
| vBMD |
Volumetric bone mineral density |
| WC |
Waist circumference |
Rationale
The steroid hormone, testosterone, plays a role in both male and female development and health. In males, testosterone is involved in the stage-specific differentiation of germ cells, spermatogenesis, and the synthesis of dihydrotestosterone (DHT) and estradiol (E2). DHT stimulates sexual differentiation of male genitalia during embryogenesis, genital maturation during puberty, and growth of pubic and facial hair (Kinter & Anekar, 2020). E2 is required in males for modulating libido, erectile function, and spermatogenesis (Schulster et al., 2016). Serum testosterone is typically solubilized by binding to the androgen-binding protein (ABP) or SHBG, which aids in regulating their transport, distribution, metabolism, and biological activity. ABP and SHBG have similar primary structure, but they differ in the types of oligosaccharides associated with them (Hammond & Bocchinfuso, 1995).
In females, testosterone is primarily synthesized and secreted in the ovaries and adrenal glands (Longcope, 1986) but some testosterone production also occurs in peripheral tissues like muscle, fat, breast, and bone (Burger, 2002). Polycystic ovary syndrome (PCOS) is one manifestation of a dysregulation of testosterone in women and is a complicated condition with a variety of metabolic, reproductive, and psychological features (Teede et al., 2018).
Primary and secondary hypogonadism are two forms of testicular hypofunction found in males. These conditions can be differentiated by the concentration of serum LH, FSH, and prolactin. Primary hypogonadism is associated with low levels of testosterone and normal to high levels of LH and FSH. Secondary hypogonadism is associated with low levels of testosterone and normal to low levels of LH and FSH (Carnegie, 2004). The anterior pituitary gland of hypothalamic-pituitary-gonadal axis releases LH and FSH, which act on the gonadal receptors to regulate testosterone production. Binding of LH to Leydig cell receptors initiates testosterone production, while testosterone secretion is further regulated by feedback inhibition (Nassar & Leslie, 2023). Males who develop hypogonadism prior to puberty often exhibit depressed secondary sex characteristics, eunuchoid stature, small testes, gynecomastia, and a small phallus. For males who develop hypogonadism after the onset of puberty, the physical findings are similar, except for a normal stature and normal phallus size (Snyder, 2024). Besides hypogonadism, testosterone production can also be affected by certain medications, chemotherapy, lifestyle, and aging (Meldrum et al., 2012; Nassar & Leslie, 2023).
In adult males, total serum testosterone levels decrease at an average rate of 1.6% per year. The concentrations of free and bioavailable testosterone decrease more rapidly, typically two to three percent annually, due to the natural increase in SHBG. By the age of 60, 20% of men will have testosterone levels below the normal range, “and the figure rises to 50% in those aged over 80” (Stanworth & Jones, 2008). Significant decrease in testosterone may result in symptoms such as fatigue, decreased libido, erectile dysfunction, depression, muscle weakness, and others. Unfortunately, these symptoms are not specific to testosterone deficiency (Bhasin et al., 2018). Low testosterone levels are associated with diabetes (Hassanabad & Fatehi, 2018), metabolic syndrome (Mohammed et al., 2018), cardiovascular disease (Corona et al., 2018; Wang et al., 2018), obesity (Molina-Vega et al., 2018), sleep apnea (Viana et al., 2017), and other disorders (Nassar & Leslie, 2023). Additionally, testosterone elevations are associated with serious conditions including tumors, hyperthyroidism, and genetic disorders such as congenital adrenal hyperplasia (Bhasin et al., 2018; Nassar & Leslie, 2023).
Within the serum, testosterone can be either free (i.e., not bound to a specific protein) or protein-bound. Only one to four percent of circulating testosterone is usually found free. SHBG binds testosterone with a high affinity whereas serum albumin, corticosteroid-binding globulin (CBG), and orosomucoid binds testosterone with a much lower affinity. “Bioavailable” testosterone refers to the amount of free testosterone and albumin-bound testosterone as indicated in the figure below (Goldman et al., 2017).
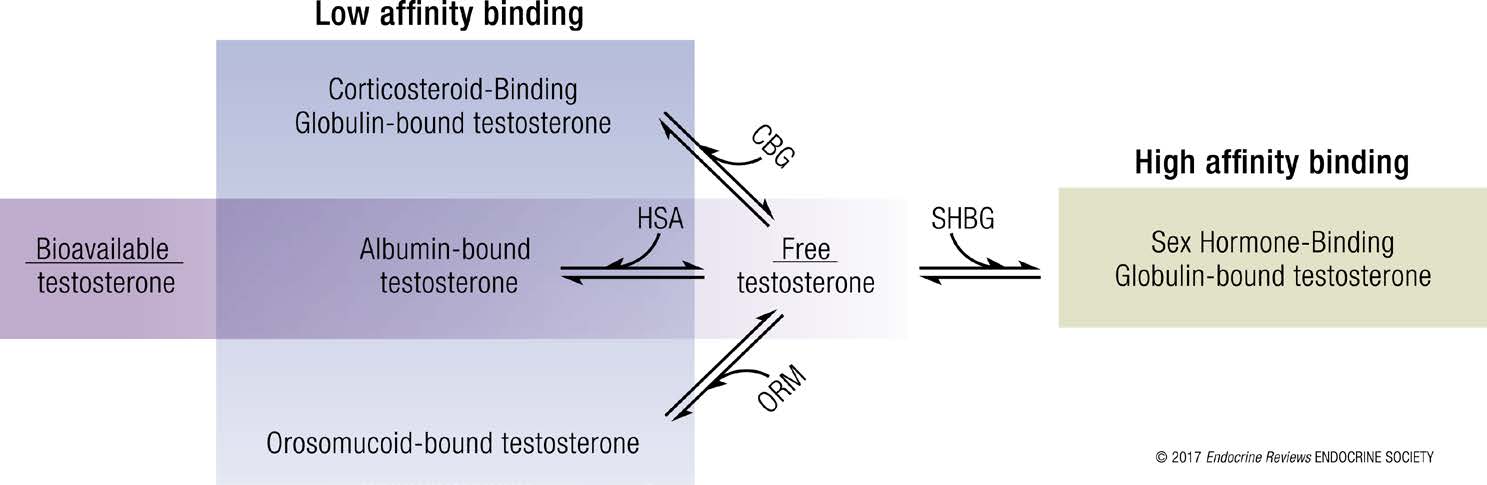
CDC Hormone Standardization (HoSt) Program-Testosterone
Serum testosterone testing can measure either total testosterone (TT) concentration, free testosterone, or bioavailable testosterone. TT can be “measured using radioimmunoassay, immunometric assays, or liquid chromatography-tandem mass spectrometry. Considerable inter-assay and inter-laboratory variability is often found in TT measurements. When 1133 laboratories using 14 different assays measured TT concentrations using the same College of American Pathologists quality control sample from a single hypogonadal man, the measured values ranged from 45 to 365 ng/dL (1.6 to 12.7 nmol/L)” (Bhasin et al., 2018).
The Centers for Disease Control and Prevention (CDC) released their analysis of TT in serum by isotope dilution-liquid chromatography-tandem mass spectrometry (ID-LC-MS/MS) in 2012. As a part of the CDC HoSt Program (CDC Hormone Standardization Program) to certify and calibrate hormone assays, the CDC monitors and validates hormone testing by laboratories and manufacturers. “Calibration is further verified by analyzing serum material with assigned reference values for total testosterone every 6 months and comparing the results obtained against predefined acceptance limit, which is ± 6.4 % from the target value” (CDC, 2012). According to CDC standards, TT using ID/HPLC/MS/MS methodology has a reportable range of 2.5 – 1000 ng/dL or 0.09 – 34.7 nM with a limit of detection of 0.36 ng/dL or 0.012 nM. As for accuracy in terms of “trueness and precision,” the CDC reports that total serum testosterone precision (%CV) ranges from 2.2% to 5.5%. The following limitation was noted: “This method was tested for total testosterone analysis in human serum and may not be suitable for other specimens such as plasma, whole blood, urine, and/or saliva. The analytical performance parameters need to be reassessed and verified when other specimen matrices are used” (CDC, 2012). A list of the assays certified by the CDC HoSt/Testosterone program can be found on the CDC’s HoSt website (CDC, 2023).
Analytical Validity
A recent study used a liquid chromatography with tandem mass spectrometry (LC-MS-MS) method to assess salivary testosterone, androstenedione, dehydroepiandrosterone, and 17-OH-progesterone. The authors state that the accuracy of this method is “between 83.0 and 106.1% for all analytes” and conclude that this “LC-MS/MS method allowed a sensitive evaluation of androgen salivary levels and represents an optimal technique to explore the relevance of a comprehensive androgen profile as measured in saliva for the study of androgen secretion modulation and activity in physiologic and pathologic states” (Mezzullo et al., 2017). However, another study compared various ELISA-based salivary testosterone assays and noted that “proportional errors between the methods calls [sic] for caution” as one of the four methods yielded no results due to malfunction (Andersson et al., 2017). Another study compared salivary testosterone measurements using immunoassays with those measured by tandem mass spectrometry. The authors conclude that the immunoassay-based methods “tended to inflate estimates of lower testosterone concentrations” (Welker et al., 2016).
Recently, van der Veen et al. (2019) developed and validated a LC-MS/MS method to establish reliable reference intervals in five plasma steroid hormones (progesterone, 17-hydroxyprogesterone, androstenedione, testosterone and dihydrotestosterone); these researchers utilized samples from 280 healthy male and female participants over a four-month period. Women taking oral contraceptive pills were found to have lower levels of 17-OH-progesterone and androstenedione; further, it was identified that hormonal biological variation was typically greater in women compared to men (van der Veen et al., 2019). Final conclusions stated that “The gender-specific determination of the reference intervals, together with the observation that the biological variation demonstrated a high degree of variation, allows interpretation of data on individual and group level for improved biochemical characterization of patients in clinical practice” (van der Veen et al., 2019).
Star-Weinstock and Dey (2019) developed an accurate and sensitive method to measure testosterone in hypogonadal adults and children of both genders; this quantification method utilized electrospray ionization (ESI)-LC-MS/MS and achieved a “sensitivity of one ng/dL from 100 µL sample volume.” The authors note that two highlights of this novel method are that this sample preparation technique “includes simultaneous protein precipitation and derivatization,” and that this TT measurement method was certified by the CDC Hormone Standardization program (Star-Weinstock & Dey, 2019).
Sun et al. (2020) developed and validated an isotope dilution ultra-performance liquid chromatography-tandem mass spectrometry method (ID-UPLC-MS/MS) to measure human serum testosterone. This method offers higher accuracy and lower variability than the traditional immunoassays, especially when measuring low testosterone levels in hypogonadism. To assess accuracy of the method, pure testosterone was added to the serum samples and the actual concentrations after two serial liquid-liquid extractions were measured. The actual concentrations were close to the female and male levels, with a recovery rate ranging from 94.32 to 108.6%. Sensitivity, specificity, and precision were also measured and met the performance criteria standards established by Clinical and Laboratory Standards Institute (Lynch, 2016) and the Hormone Standardization Program of the Center for Disease Control (Yun et al., 2012). “Moreover, the [ID-UPLC-MS/MS] method exhibited a good consistency between low and high concentrations of testosterone. In addition, the method required a simple sample preparation and a small sample volume, therefore it may be suitable for routine clinical practice” (Sun et al., 2020).
The Centers for Disease Control and Prevention (CDC) reviewed testosterone testing in 2014 in a group of 6746 participants of various age groups and both sexes (Vesper et al., 2014). The positive bias identified by steroid analyte testing indicated that the test was measuring additional compounds (and not only the analyte in question). The authors concluded that “although technologies for steroid hormone measurement have advanced significantly, measurement variability within and across laboratories has not improved accordingly. … Within-assay variability for current assays is generally high, especially at low analyte concentrations” (Vesper et al., 2014).
Testosterone and other hormones (AMH, FSH, LH, free androgen index (FAI), prolactin, estradiol) have been used for the clinical diagnosis of polycystic ovary syndrome (PCOS). In a study completed by Khashchenko et al. (2020), 130 girls with PCOS had the accuracy and specificity of hormonal testing assessed and cutoffs for the most significant hormone indicators of PCOS diagnosis in adolescents were identified. The authors found that “Levels of testosterone > 1.15 nmol/L, androstenedione > 11.45 ng/mL, and LH/FSH ratio > 1.23 also showed high sensitivity of 63.2–78.2% and specificity of 84.4% – 93.7% in PCOS diagnosis in the studied sample of girls” (Khashchenko et al., 2020). The combined use of either four thresholds (AMH, FAI, testosterone, androstenedione, LH/FSH ratio as previously stated) yielded a diagnostic accuracy of 90.2% – 91.6% in predicting PCOS in adolescents (Khashchenko et al., 2020).
Dalmiglio et al. (2024) evaluated the Vermeulen formula as less expensive and more accessible method of assessing FT than equilibrium dialysis or ultrafiltration. The study included 190 patients, all of whom received FT measurements through both direct immunoluminometric assay and the Vermeulen formula. The authors claim that “the calculated method employing the Vermeulen formula was considered the gold standard.” The authors noted that the sensitivity was lower in females, which they claim could be because of a potential proportional bias and the low number of true positive cases. The authors concluded that “the direct method exhibited comparable performance to the calculated method, but caution should be exercised when interpreting results, particularly in females” (Dalmiglio et al., 2024).
Clinical Utility and Validity
Equilibrium dialysis is the gold standard for determining free serum testosterone. Unfortunately, it is technically difficult and has limited availability. Compared to other less accurate methods, it is expensive. It relies on the accuracy and precision of TT determination. In equilibrium dialysis, a semipermeable membrane is used to retain the bound testosterone on one side of the membrane while the free testosterone equilibrates between the two sides. It is dependent on environmental conditions including pH, ionic strength, and temperature; in fact, steroids, such as testosterone, can bind up to 2.5 times higher at 4◦C than at 37◦C. One study shows that increasing the temperature from 37◦C to 41◦C increased the free cortisol level by approximately 80% (Goldman et al., 2017).
Immunoassays to measure free and bioavailable testosterone are inaccurate. The Endocrine Society urges the use of medically accepted algorithms that rely on TT, SHBG, and/or albumin to estimate serum free testosterone (Bhasin et al., 2018). Multiple algorithms have been published (Sartorius et al., 2009; Vermeulen et al., 1999; Zakharov et al., 2015). The recent allosteric model proposed by Zakharov and colleagues models the binding of testosterone as a multi-step, dimeric process. This allosteric model has “close correspondence with those measured using equilibrium dialysis” (Zakharov et al., 2015).
A 2017 international study comprised of multiple cohorts with healthy, non-obese males attempted to “derive standardized, age-specific reference ranges” for circulating testosterone; it was stated that “a substantial proportion of intercohort variation in testosterone levels is due to assay differences” (Travison et al., 2017). Further, the issue in developing standards for circulating testosterone due to variation in body mass and comorbidities was also noted. “Another unresolved issue relates to whether the reference sample should include only the healthy nonobese men or whether it should include the entire population of men 19 to 39 years. Obesity and comorbid conditions affect circulating total testosterone concentrations; therefore, inclusion of obese men with comorbid conditions could distort the reference ranges. Whether the reference ranges generated in nonobese men are appropriate for use in obese men deserves further investigation. Even though men with known diagnoses of conditions or diseases associated with hypogonadism were excluded, it is possible a small percentage of individuals in these cohorts may be hypogonadal” (Travison et al., 2017).
Shukla et al. (2018) organized a cross-sectional study to measure the relationship between prostatic specific antigen (PSA) and serum testosterone levels in both healthy men and men with partial androgen deficiency (PADAM); a total of 255 men participated in this study. “Mean total testosterone and serum PSA was 9.35 ± 1.33 nmol/L, 1.96 ± 0.76 ng/mL in males with PADAM and 15.30 ± 1.95 nmol/L, 1.85 ± 0.73 ng/mL respectively in males without PADAM. No significant relationship was observed between serum PSA and serum testosterone levels among healthy males irrespective of PADAM” (Shukla et al., 2018). Results from this study suggest that PSA values do not need to be adjusted “for biopsy decisions according to testosterone levels” (Shukla et al., 2018).
In a retrospective cohort study, eighty-five severely hypogonadal men were observed for changes in serum prostate specific antigen (PSA) concentrations during testosterone treatment for 18 months. The Endocrine Society clinical guidelines recommend measuring PSA in hypogonadal men over the age of 50 at three months and twelve months after starting testosterone therapy and urologic referral if serum PSA > 1.4 ng/mL above baseline or to an absolute value > four ng/mL (Bhasin et al., 2018). Studies have been performed in men with mild to moderate hypogonadism which reported smaller increases in serum PSA concentrations during testosterone treatment; however, no studies have reported serum PSA changes in response to testosterone treatment of severely hypogonadal men. In this study, testosterone treatment “increased the median serum testosterone concentration from 36 ng/dL at baseline to 395 ng/dL at 6 – 18 months. This treatment resulted in a median increment in PSA above baseline of 0.70 ng/mL at 6 – 18 months … 31% of men had increases in PSA > 1.4 ng/mL; and 13% of men reached absolute PSA concentrations > four ng/mL” (Sachdev et al., 2020). The authors suggest that “testosterone treatment of severely hypogonadal men often increases PSA above the commonly accepted thresholds for urologic referral [and] that future clinical guidelines for the expected PSA response to testosterone replacement reflect the degree of hypogonadism” (Sachdev et al., 2020).
A total of nine years of registry data, comprised of 650 patients with hypogonadism, was analyzed to determine the impact of long-term intramuscular testosterone treatment (1000 mg every 10 – 12 weeks) (Zitzmann et al., 2019). Serum testosterone concentrations were found to increase “from 5.7 ± 2.3 nmol/L to 19.4 ± 2.8 nmol/L in men with classical hypogonadism and from 7.8 ± 2.4 nmol/L to 19.2±3.1 nmol/L in men with functional hypogonadism”; final conclusions suggest that patients with the functional form of hypogonadism may benefit the most from testosterone treatment as “men with functional hypogonadism were more likely to lose ten percent weight and five percent of waist circumference (WC) than men with classical hypogonadism” (Zitzmann et al., 2019). Men with functional hypogonadism were also more likely to be obese at the start of the study.
Cauley et al. (2021) performed a study to examine the effect of testosterone treatment on TBS. “Two hundred and eleven men were enrolled in Bone Trial of the Testosterone Trials. Of these, 197 men had two repeat TBS and vBMD measurements; 105 men were allocated to receive testosterone, and 92 men to placebo for one year. TBS, a BMD, and vBMD were assessed at baseline and month 12.” The results of this study report that there was no difference in the percent change in TBS by randomized group. They saw a 1.6% (95% confidence intervals (CI) 0.2–3.9) change in the testosterone group and a 1.4% (95% CI −0.2, 3.1) change in the placebo group. In contrast, they saw a six percent increase in vBMD (95% CI4.5 – 7.5) in the testosterone group as compared to only a 0.4% vBMD change (95% CI−1.65 – 0.88) in the placebo groups (Cauley et al., 2021). As a result, the authors concluded that TBS was not clinically useful in monitoring the one year effect of testosterone treatment on the bone structure in older hypogonadal men (Cauley et al., 2021).
Stern and Casto (2024) studied the differences in salivary testosterone levels across the menstrual cycle. A total of 339 people with a menstrual cycle and confirmed ovulation were included. Salivary testosterone was measured with LC-MS/MS four times across the mid-cycle ovulatory window during the luteal phase. “Within-subject analysis revealed a significant but small pattern of a mid-cycle peak and a luteal decrease at the aggregate level.” The authors note that at the individual level, there was “substantial variability between the direction and magnitude of the testosterone-cycle pattern.” The authors conclude that “salivary testosterone levels show a small trend towards a mid-cycle peak compared to the earlier follicular phase and the later luteal phase of the menstrual cycle when looking at the aggregate across all participants,” but overall, “menstrual patterns of testosterone appear subtle and not systematic across individuals” (Stern & Casto, 2024).
Maimoun et al. (2011) studied the diagnosis process of 5-alpha reductase deficiency. The study included 55 patients with srd5A2 gene mutations. The authors found a “wide spectrum” of phenotypes, including clitoromegaly in 49.1% of participants, microphallus with various degrees of hypospadias in 32.7% of participants, female external genitalia in 7.3% of participants and isolated micropenis in 3.6% of participants. Overall, “over 72% of patients were considered for 5α-reductase deficiency diagnosis when the testosterone/dihydrotestosterone cutoff was 10” (Maimoun et al., 2011).
Imperato-McGinley et al. (1986) studied the clinical criteria used to diagnosis three infants with 5-alpha reductase deficiency. Initially, “basal plasma testosterone to dihydrotestosterone ratios were significantly elevated in two of the three affected infants, and increased markedly in all three infants after administration of hCG.” The authors note that urinary etiocholanolone to androsterone ratios could not be accurately measured in infants, so the diagnosis was confirmed by using gas chromatography/mass spectrometry to measure urinary tetrahydrocortisol (THF) to 5 alpha-tetrahydrocortisol (5 alpha-THF) ratios. “The affected infants had THF/5 alpha-THF ratios comparable to ratios in adult carrier males and significantly lower than ratios in adult homozygotes.” The authors concluded that 5 alpha reductase is detectable at infancy (Imperato-McGinley et al., 1986).
The Endocrine Society (ES)
Androgen Deficiency Testosterone Therapy
The ES, in updated 2018 guidelines concerning testosterone therapy and hypogonadism in males, summarized their recommendations with respect to testosterone testing with the following:
- Recommendation 1.1: “We recommend diagnosing hypogonadism in men with symptoms and signs of testosterone deficiency and unequivocally and consistently low serum total testosterone and/or free testosterone concentrations (when indicated).” (Level 1+++)
- Recommendation 1.2: “We recommend against routine screening of men in the general population for hypogonadism.” (Level 1++)
- Recommendation 1.3: “In men who have hypogonadism, we recommend distinguishing between primary (testicular) and secondary (pituitary–hypothalamic) hypogonadism by measuring serum luteinizing hormone and follicle-stimulating hormone concentrations.” (Level 1+++)
- Recommendation 1.4: “In men with hypogonadism, we suggest further evaluation to identify the etiology of hypothalamic, pituitary, and/or testicular dysfunction.” (Level 2++)
- Recommendation 3.1: “In hypogonadal men who have started testosterone therapy, we recommend evaluating the patient after treatment initiation to assess whether the patient has responded to treatment, is suffering any adverse effects, and is complying with the treatment regimen.” (Ungraded Good Practice Statement)
Within the explanations and technical comments of the recommendations, the ES specifically states, “Clinicians should not use direct analog-based free testosterone immunoassays, as they are inaccurate.” Moreover, recommendations state that serum total testosterone testing is preferred and should be performed on two separate days after fasting since testosterone concentrations can vary due to many circumstances. For men who initially test low TT, who test near the lower limit, or who have conditions associated with altering SHBG, then free testosterone can be measured either by using an accepted algorithm based on the TT, SHBG, and albumin concentrations or by direct equilibrium dialysis methods rather than the use of immunoassays. As for bioavailable testosterone testing, the ES states, “Measuring bioavailable [testosterone] concentrations using ammonium sulfate precipitation is technically challenging. Furthermore, there are no detailed studies (similar to those described previously that relate FT [free testosterone] concentrations to manifestations of [testosterone] deficiency) that use bioavailable [testosterone] concentrations.” Men beginning hormone replacement therapy should have their serum testosterone and hematocrit levels measured initially to establish a baseline and then, depending on the therapy, have the levels measured again three to six months later. While on testosterone therapy, the TT and hematocrit levels should be checked annually thereafter. Concerning secondary hypogonadism, serum prolactin and either serum ferritin or iron saturation measurements are recommended to check for the possibility of reversibility of the condition. The testing algorithm also recommends testing serum LH and FSH to differentiate primary and secondary hypogonadism. The algorithm for testing for hypogonadism is shown in the figure below (Bhasin et al., 2018):
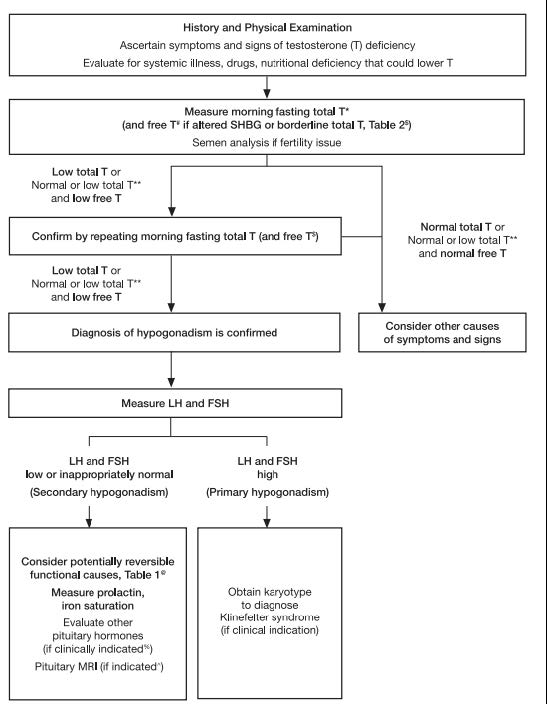
Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline
Testosterone testing in addition to other endocrine laboratory tests is recommended as part of an initial endocrine assessment for women with clinical hyperandrogenism in the evaluation of suspected functional hypothalamic amenorrhea (FHA). FHA is a condition of anovulation, in which the ovary fails to release an egg during the menstrual cycle and has been correlated with stress, weight loss, and excessive exercise (Gordon et al., 2017).
Polycystic Ovary Syndrome (PCOS)
Relative to the diagnosis of PCOS, the ES identifies three criteria that may be evaluated: androgen excess, ovulatory dysfunction, and polycystic ovaries. Two of the three criteria are sufficient for diagnosis, and if both clinical criteria are met, they do not recommend testing for androgen excess. Androgen excess is characterized by elevated serum androgen levels such as elevated total, bioavailable, or free serum testosterone levels. Considering that serum testosterone levels are variable and there is poor standardization of assays, the Task Force recommends familiarity with local assays and does not define an absolute level that is diagnostic of PCOS or other causes of hyperandrogenism (Legro et al., 2013).
Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons
The ES published guidelines suggesting that testosterone level monitoring is suggested at baseline and every 6 – 12 months during suppression of puberty treatment protocol in gender-dysphoric/gender-incongruent persons. The laboratory monitoring of testosterone levels is also suggested at baseline and every 6 – 12 months during induction of puberty protocol. Measurement of serum testosterone levels is suggested every three months until levels are in the normal physiologic male range during the monitoring of transgender males on gender-affirming hormone therapy. Testosterone testing is also needed midway between injections for monitoring of testosterone enanthate/cypionate injections, alternatively peak and trough levels could be measured to ensure levels remain in the normal male range. For parenteral testosterone undecanoate, testosterone should be measured just before the following injection. For transdermal testosterone, the testosterone level can be measured no sooner than after one week of daily application (at least 2h after application). For monitoring transgender females on gender-affirming hormone therapy, measurement of serum testosterone is indicated every three months (Hembree et al., 2017).
The American College of Obstetricians and Gynecologists
In 2018, the ACOG released guidelines on the clinical management of polycystic ovary syndrome (PCOS). In its suggested evaluation of patients with PCOS, the ACOG recommends having a physical, laboratory testing, and an ultrasound examination to confirm the polycystic ovaries. With regards to hormone testing, it includes “documentation of biochemical hyperandrogenemia” by “total testosterone and sex-hormone binding globulin or bioavailable and free testosterone,” but notes to conduct testing that would exclude other causes of hyperandrogenism, such as thyroid dysfunction and hyperprolactinemia. ACOG includes TSH, prolactin, and 17-hydroxyprogesterone as hormones to measure to exclude other causes. The ACOG (2018b) also acknowledges that “there is no standardized testosterone assay in the United States and the sensitivity and reliability in the female ranges are often poor.”
Regarding Müllerian Agenesis, ACOG writes that the initial evaluation of a patient without a uterus “may include the following laboratory tests: testosterone level, FSH level, and karyotype” (ACOG, 2018a).
In 2019, ACOG released a guideline regarding the “screening and management of the hyperandrogenic adolescent.” In it, they state that the diagnosis of hyperandrogenism can be based on clinical symptoms or measurement of serum androgens. However, they recommend against monitoring serum androgens. This guideline was reaffirmed in 2024.
The ACOG recommends identifying clinical symptoms of androgen excess during the initial evaluation. In the proposed algorithm for evaluation, ACOG recommends two separate batteries of hormone tests depending on type of menses. For regular menses, ACOG lists free and TT, DHEAS (dehydroepianandrosterone sulphate), and 17OHP (17-α-hydroxyprogesterone) as hormones that may be tested. For irregular menses, ACOG lists prolactin, LH, FSH, TSH, and the three previously mentioned hormones. ACOG also notes that PCOS may be one of the diagnoses if both androgen excess and irregular menses are identified (ACOG, 2019). This guideline was reaffirmed in 2024.
American Urological Association (AUA)
The AUA published guidelines concerning the evaluation and management of testosterone deficiency in 2018. Five recommendations are given concerning the diagnosis of testosterone deficiency:
- “Clinicians should use a total testosterone level below 300 ng/dL as a reasonable cut-off in support of the diagnosis of low testosterone. (Moderate Recommendation; Evidence Level: Grade B)
- The diagnosis of low testosterone should be made only after two total testosterone measurements are taken on separate occasions with both conducted in an early morning fashion. (Strong Recommendation; Evidence Level: Grade A)
- The clinical diagnosis of testosterone deficiency is only made when patients have low total testosterone levels combined with symptoms and/or signs. (Moderate Recommendation; Evidence Level: Grade B)
- Clinicians should consider measuring total testosterone in patients with a history of unexplained anemia, bone density loss, diabetes, exposure to chemotherapy, exposure to testicular radiation, HIV/AIDS, chronic narcotic use, male infertility, pituitary dysfunction, and chronic corticosteroid use even in the absence of symptoms or signs associated with testosterone deficiency. (Moderate Recommendation; Evidence Level: Grade B)
- The use of validated questionnaires is not currently recommended to either define which patients are candidates for testosterone therapy or to monitor symptom response in patients on testosterone therapy. (Conditional Recommendation; Evidence Level: Grade C)”
Other recommendations by the AUA concerning adjunctive testing in males include the following:
6. “In patients with low testosterone, clinicians should measure serum luteinizing hormone levels (Strong Recommendation; Evidence Level: Grade A)
7. Serum prolactin levels should be measured in patients with low testosterone levels combined with low or low/normal luteinizing hormone levels (Strong Recommendation; Evidence Level: Grade A)
8. Patients with persistently high prolactin levels of unknown etiology should undergo evaluation for endocrine disorders (Strong Recommendation; Evidence Level: Grade A)
9. Serum estradiol should be measured in testosterone deficient patients who present with breast symptoms or gynecomastia prior to the commencement of testosterone therapy. (Expert Opinion)
10. Men with testosterone deficiency who are interested in fertility should have a reproductive health evaluation performed prior to treatment. (Moderate Recommendation; Evidence Level: Grade B)
11. Prior to offering testosterone therapy, clinicians should measure hemoglobin and hematocrit and inform patients regarding the increased risk of polycythemia. (Strong Recommendation; Evidence Level: Grade A)
12. PSA should be measured in men over 40 years of age prior to commencement of testosterone therapy to exclude a prostate cancer diagnosis. (Clinical Principle)” (Mulhall et al., 2014).
American Academy of Pediatrics (AAP) — Choosing Wisely Initiative
As a part of the Five Things Physicians and Patients Should Question series of the Choosing Wisely initiative of the American Board of Internal Medicine (ABIM) foundation, the AAP states the following: “Avoid ordering LH and FSH and either estradiol or testosterone for children with pubic hair and/or body odor but no other signs of puberty.” Further, “premature adrenarche is usually the diagnosis and does not involve activation of the pituitary- gonadal axis but is due to an early increase in adrenal androgens. DHEA-S levels are elevated for age but do not alter the management of this common and generally benign condition” (AAP, 2017).
European Academy of Andrology (EAA)
The EAA published guidelines concerning management of bone health in males and testing in Andrology, a journal jointly published by the EAA and the American Society of Andrology. Recommendations include the following:
- “We recommend having serum total testosterone measured twice on a morning blood sample.” (Level 1+++)
- “We recommend measuring again total testosterone and SHBG if only a single measurement documenting low testosterone is available. LH and prolactin are useful to better characterize hypogonadism.” (Level 1+++)
- “We do not recommend routine measurement of serum estradiol.” (Level 1++)
- “We suggest measuring estradiol only when a validated mass spectrometry-based method is available and in rare cases in which severe estrogen deficiency is suspected.” (Level 2++)
Within the evidence and rationale behind the recommendations, the EAA goes on to state, “We suggest using calculated free testosterone when needed, based on the measurement of total serum testosterone, SHBG, and albumin. ... It can easily be obtained using online available calculators (see Appendix 2 [of (Rochira et al., 2018)] for Web links). Commercially available kits for direct measurement of free testosterone should not be used due to their poor accuracy and reliability” (Rochira et al., 2018). Concerning other hormones, the EEA states, “all patients with documented low serum testosterone consulting with hypogonadal symptoms should receive a biochemical evaluation of their gonadal status, with measurement of serum total testosterone, SHBG, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and prolactin” (Rochira et al., 2018).
The EAA also published clinical practice guidelines regarding gynecomastia evaluation and management. The EAA recommended testing several hormones for gynecomastia including “testosterone (T), estradiol (E2), sex hormone-binding globulin (SHBG), luteinizing hormone (LH), follicular stimulating hormone (FSH), thyroid stimulating hormone (TSH), prolactin, human chorionic gonadotropin (hCG), alpha-fetal protein (AFP), liver and renal function tests” (Kanakis et al., 2019).
The EAA recently published clinical practice guidelines on investigation, treatment, and monitoring of functional hypogonadism in males to provide certain recommendations:
- “We recommend against universal screening for hypogonadism in middle-aged or older men, by structured interviews or questionnaires and/or random total T measurements.
- We recommend that the clinical diagnosis of functional hypogonadism should be confirmed by measurement of serum total T with a well validated assay on fasting morning (before 11 am) blood samples obtained on two different days.
- Functional hypogonadism should be diagnosed only after exclusion of organic causes of hypogonadism. In addition, to morning total T, luteinizing hormone (LH) should be measured in all patients with suspected functional hypogonadism to differentiate between the primary and secondary causes.
- We recommend either measuring or calculating free T (fT), in addition to total T, in patients with conditions that alter sex hormone-binding globulin (SHBG) and when total T concentrations are in the borderline range (~8-12 nmol/L) if the clinical suspicion of hypogonadism is strong” (Corona et al., 2020).
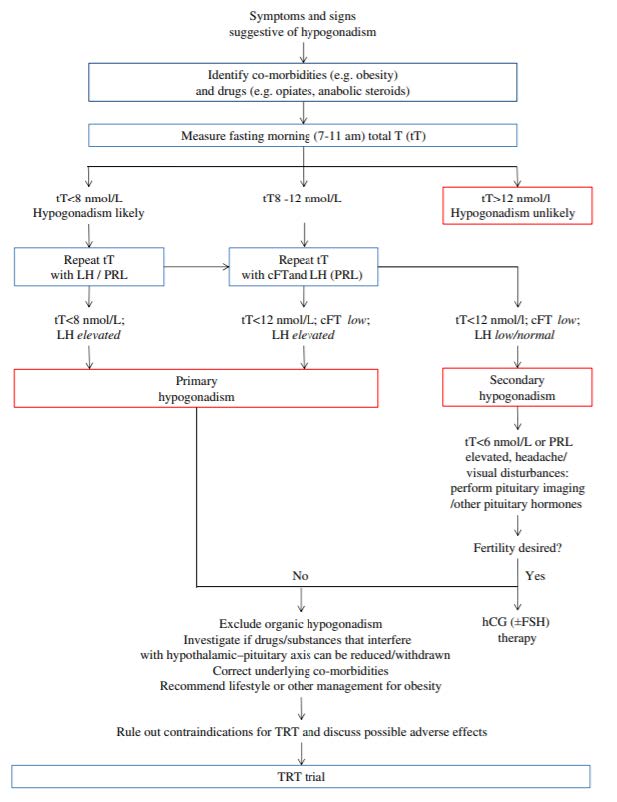
 Above, is a proposed flow chart to diagnose and manage functional hypogonadism (Corona et al., 2020).
Above, is a proposed flow chart to diagnose and manage functional hypogonadism (Corona et al., 2020).
Canadian Urological Association (CUA) and Canadian Society of Endocrinology and Metabolism (CSAM)
The CUA and CSAM endorsed joint guidelines published in the CMAJ in 2015. The following recommendations were given concerning hormone testing in males for testosterone deficiency syndrome (Morales et al., 2015):
- “We recommend a thorough history and physical examination, instead of the exclusive reliance on standard questionnaires, to identify patients requiring biochemical testing (strong recommendation; moderate-quality evidence).”
- “The initial biochemical test should be total testosterone level measured in serum samples taken in the morning; determinations of bioavailable testosterone or free testosterone should be restricted to patients with equivocally low total testosterone levels (strong recommendation; high-quality evidence).”
- “We recommend that sample collection for testosterone measurement occur between seven am and eleven am, or within three hours after waking in the case of shift workers (strong recommendation; moderate-quality evidence).”
- “Testosterone levels should be measured with the use of testosterone assays traceable to internationally recognized standardized reference material; commercial assays should be certified by the testosterone standardization program of the US Centers for Disease Control and Prevention (strong recommendation; high-quality evidence).”
- “Measurement of sex hormone-binding globulin with calculated free or bioavailable testosterone should be restricted to men with symptoms of testosterone deficiency and equivocally low testosterone levels (strong recommendation; moderate-quality evidence).”
- “We recommend investigation for secondary or reversible causes of hypogonadism in all men with testosterone deficiency syndrome (strong recommendation; moderate-quality evidence).”
- “We recommend investigation for testosterone deficiency syndrome and treatment with testosterone in men with anemia or sarcopenia of undetermined origin (strong recommendation; moderate-quality evidence).”
- “We recommend assessment of response and adverse effects at three and six months after onset of therapy (strong recommendation; high-quality evidence).”
- “Testosterone levels should be assessed at three and six months after onset of therapy and then annually thereafter if stable (weak recommendation; low-quality evidence).”
European Association of Urology (EAU)
In the 2014 EAU guidelines concerning the treatment of castration-resistant prostate cancer, the EAU states, “Follow-up after ADT should include analysis of PSA and testosterone levels, and screening for cardiovascular disease and metabolic syndrome” (Heidenreich et al., 2014).
The EAU released guidelines on sexual and reproductive health and expressed the following recommendations for diagnosis of hypogonadism:
- “Check for concomitant diseases, drugs and substances that can interfere with testosterone production/action.
- Measure total testosterone in the morning (between 07.00 and 11.00 hours) and in the fasting state, with a reliable laboratory assay.
- Repeat total testosterone on at least two separate occasions when < 12 nmol/L and before starting testosterone therapy.
- Use 12 nmol/L total testosterone (3.5 ng/mL) as a reliable threshold to diagnose late onset hypogonadism (LOH).
- Measure sex hormone-binding globulin and free-testosterone calculation when indicated.
- Analyse luteinising hormone and follicle-stimulating hormone serum levels to differentiate between the different types of hypogonadism.
- Measure prolactin (PRL) levels if evidence of low sexual desire (or other suggestive signs/ symptoms) and secondary hypogonadism is present.
- Perform pituitary magnetic resonance imaging (MRI) in secondary hypogonadism, with elevated PRL or symptoms specific of a pituitary mass and/or presence of other anterior pituitary hormone deficiency.
- Perform pituitary MRI in secondary severe hypogonadism (total testosterone < 6 nmol/L)..
- Screen for late onset hypogonadism (LOH) only in symptomatic men.
- Do not use structured interviews and self-reported questionnaires for systematic screening for LOH as they have a low specificity.”
The EAU recommends that the standard and most accurate method for testosterone serum testing is liquid chromatography-tandem mass spectrometry (LC-MS/MS). Standardized automated platform immuno-assays are reliable techniques to measure testosterone; however, only LC-MS/MS can provide an accurate measurement of FT (fT) levels. When diagnosing late-onset hypogonadism, the EAU recommends measuring fasting and morning (7 – 11 a.m.) TT, noting to “(consider PRL measurement if low desire or other suggestive symptoms are present,” “consider SHBG and free-T calculations when indicated,” consider LH when T deficiency pathophysiology must be investigated.” There is uncertainty as to what threshold of fT level indicates hypogonadism, but some data indicates that fT levels below 225 pmol/L is associated with hypogonadism (Salonia et al., 2024).
National Comprehensive Cancer Network
Within the algorithm concerning the systemic therapy for castration-naïve disease, the NCCN says to “document castrate level of testosterone if clinically indicated” when assessing progression along with the physical exam and PSA every three to six months. The NCCN also states to “continue ADT [androgen deprivation therapy] to maintain castrate serum levels of testosterone (< 50 ng/dL).” Additional recommendations state, “close monitoring of PSA and testosterone levels and possibly imaging is required when using intermittent ADT, especially during off-treatment periods, and patients may need to switch to continuous ADT upon signs of disease progression” (NCCN, 2024b).
The NCCN also published some guidance regarding assessment of hormones for neuroendocrine and adrenal tumors. For pituitary tumors, they list serum prolactin and LH/FSH; for “suspected or confirmed adrenocortical carcinoma”, they list "screen for hypercortisolemia (± Cushing syndrome) and primary aldosteronism” and “adrenal androgens (DHEAS, androstenedione, testosterone, 17-hydroxyprogesterone)”; for hypercortisolemic Cushing’s Syndrome, they list “screen for hypercortisolemia (± Cushing syndrome) with 1 of the following tests: 1 mg overnight dexamethasone suppression test, 2 – 3 midnight salivary cortisols, [or] 24-hour urinary free cortisol” and “plasma ACTH [adrenocorticotropic hormone] in AM if confirmed hypercortisolemia (± Cushing syndrome)” (NCCN, 2024a).
International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) & PanCareSurFup (PCSF) Consortium
Within the guidelines and recommendations issued in 2017 by the IGHG and the PCSF Consortium for patients with possible impaired spermatogenesis, it is recommend that “Clinical measurement of testicular volume and of follicle-stimulating hormone and inhibin B might be reasonable for the identification of impaired spermatogenesis in survivors treated with potentially gonadotoxic chemotherapy or radiotherapy potentially exposing the testes in whom semen analysis has been declined or is not possible and who desire assessment about possible future fertility. Be aware of the diagnostic limitations of these tests that may result in false positives or false negatives (level B evidence).” With respect to patients with possible testosterone deficiency, “Measurement of testosterone concentration in an early morning blood sample at clinically appropriate intervals is reasonable in post pubertal survivors treated with radiotherapy potentially exposing the testes to 12 Gy or more or with TBI (expert opinion). In the presence of clinical signs of hypogonadism, or of previous low-normal or borderline testosterone concentrations, or if it is not possible to obtain an early morning blood sample, it is reasonable to measure luteinising hormone concentration in addition to testosterone (expert opinion)” (Skinner et al., 2017).
American Society of Reproductive Medicine (ASRM)
The ASRM, in collaboration with the Society for Male Reproduction and Urology, released a committee opinion on the diagnostic evaluation of sexual dysfunction in the male partner in the setting of infertility. The publication recommends the following for the detection of erectile dysfunction: “A physical examination should include blood pressure and the calculation of body mass index, as well as an assessment for signs of testosterone deficiency. Morning serum testosterone should be assayed, as should glucose and hemoglobin A1c levels, as well as lipid profile measurements, as indicated” (ASRM, 2023).
References
- AAP. (2017, 10/02/2017). Avoid ordering LH and FSH and either estradiol or testosterone for children with pubic hair and/or body odor but no other signs of puberty. ABIM Foundation. Retrieved 10/19/2018 from https://www.aafp.org/pubs/afp/collections/choosing-wisely/352.html
- ACOG. (2018a). ACOG Committee Opinion No. 728: Müllerian Agenesis: Diagnosis, Management, And Treatment. Obstet Gynecol, 131(1), e35-e42. https://doi.org/10.1097/aog.0000000000002458
- ACOG. (2018b). ACOG Practice Bulletin No. 194: Polycystic Ovary Syndrome. Obstet Gynecol, 131(6), e157-e171. https://doi.org/10.1097/aog.0000000000002656
- ACOG. (2019). Screening and Management of the Hyperandrogenic Adolescent: ACOG Committee Opinion, Number 789. Obstet Gynecol, 134(4), e106-e114. https://doi.org/10.1097/aog.0000000000003475
- Andersson, C. R., Bergquist, J., Theodorsson, E., & Strom, J. O. (2017). Comparisons between commercial salivary testosterone enzyme-linked immunosorbent assay kits. Scand J Clin Lab Invest, 77(8), 582-586. https://doi.org/10.1080/00365513.2017.1339231
- ASRM. (2023). Diagnostic evaluation of sexual dysfunction in the male partner in the setting of infertility: a committee opinion. Fertil Steril, 120(5), 967-972. https://doi.org/10.1016/j.fertnstert.2023.07.001
- Bhasin, S., Brito, J. P., Cunningham, G. R., Hayes, F. J., Hodis, H. N., Matsumoto, A. M., Snyder, P. J., Swerdloff, R. S., Wu, F. C., & Yialamas, M. A. (2018). Testosterone Therapy in Men With Hypogonadism: An Endocrine Society* Clinical Practice Guideline. The Journal of Clinical Endocrinology & Metabolism, 103(5), 1715-1744. https://doi.org/10.1210/jc.2018-00229
- Burger, H. G. (2002). Androgen production in women. Fertil Steril, 77 Suppl 4, S3-5. https://doi.org/10.1016/s0015-0282(02)02985-0
- Carnegie, C. (2004). Diagnosis of hypogonadism: clinical assessments and laboratory tests. Rev Urol, 6 Suppl 6(Suppl 6), S3-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1472884/
- Cauley, J. A., Ellenberg, S. S., Schwartz, A. V., Ensrud, K. E., Keaveny, T. M., & Snyder, P. J. (2021). Effect of testosterone treatment on the trabecular bone score in older men with low serum testosterone. Osteoporos Int, 32(11), 2371-2375. https://doi.org/10.1007/s00198-021-06022-1
- CDC. (2012). Total Testosterone. Centers for Disease Control and Prevention. Retrieved 10/23/2018 from https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/TST_H_MET_Total%20Testosterone.pdf
- CDC. (2023, 03/09/2023). HoSt/VDSCP Certified Participants. Centers for Disease Control and Prevention. https://www.cdc.gov/clinical-standardization-programs/php/hormones/list-of-hormone-certified-assays.html
- Corona, G., Goulis, D. G., Huhtaniemi, I., Zitzmann, M., Toppari, J., Forti, G., Vanderschueren, D., & Wu, F. C. (2020). European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males. Andrology, 8(5), 970-987. https://doi.org/10.1111/andr.12770
- Corona, G., Rastrelli, G., Di Pasquale, G., Sforza, A., Mannucci, E., & Maggi, M. (2018). Endogenous Testosterone Levels and Cardiovascular Risk: Meta-Analysis of Observational Studies. J Sex Med, 15(9), 1260-1271. https://doi.org/10.1016/j.jsxm.2018.06.012
- Dalmiglio, C., Bombardieri, A., Mattii, E., Sestini, F., Fioravanti, C., Castagna, M. G., Fiorini, M., Dotta, F., & Cantara, S. (2024). Analytical performance of free testosterone calculated by direct immunoluminometric method compared with the Vermeulen equation: results from a clinical series. Hormones (Athens), 23(2), 313-319. https://doi.org/10.1007/s42000-023-00522-x
- Gill-Sharma, M. K. (2018). Testosterone Retention Mechanism in Sertoli Cells: A Biochemical Perspective. Open Biochem J, 12, 103-112. https://doi.org/10.2174/1874091X01812010103
- Goldman, A. L., Bhasin, S., Wu, F. C. W., Krishna, M., Matsumoto, A. M., & Jasuja, R. (2017). A Reappraisal of Testosterone's Binding in Circulation: Physiological and Clinical Implications. Endocr Rev, 38(4), 302-324. https://doi.org/10.1210/er.2017-00025
- Gordon, C. M., Ackerman, K. E., Berga, S. L., Kaplan, J. R., Mastorakos, G., Misra, M., Murad, M. H., Santoro, N. F., & Warren, M. P. (2017). Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab, 102(5), 1413-1439. https://doi.org/10.1210/jc.2017-00131
- Hammond, G. L., & Bocchinfuso, W. P. (1995). Sex hormone-binding globulin/androgen-binding protein: steroid-binding and dimerization domains. J Steroid Biochem Mol Biol, 53(1-6), 543-552. https://doi.org/10.1016/0960-0760(95)00110-l
- Hassanabad, M. F., & Fatehi, M. (2018). Androgen Therapy in Male Patients Suffering from Type 2 Diabetes: A Review of Benefits and Risks. Curr Diabetes Rev. https://doi.org/10.2174/1573399814666180731125724
- Heidenreich, A., Bastian, P. J., Bellmunt, J., Bolla, M., Joniau, S., van der Kwast, T., Mason, M., Matveev, V., Wiegel, T., Zattoni, F., & Mottet, N. (2014). EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol, 65(2), 467-479. https://doi.org/10.1016/j.eururo.2013.11.002
- Hembree, W. C., Cohen-Kettenis, P. T., Gooren, L., Hannema, S. E., Meyer, W. J., Murad, M. H., Rosenthal, S. M., Safer, J. D., Tangpricha, V., & T'Sjoen, G. G. (2017). Endocrine Treatment of Gender-Dysphoric/Gender-Incongruent Persons: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab, 102(11), 3869-3903. https://doi.org/10.1210/jc.2017-01658
- Imperato-McGinley, J., Gautier, T., Pichardo, M., & Shackleton, C. (1986). The diagnosis of 5 alpha-reductase deficiency in infancy. J Clin Endocrinol Metab, 63(6), 1313-1318. https://doi.org/10.1210/jcem-63-6-1313
- Kanakis, G. A., Nordkap, L., Bang, A. K., Calogero, A. E., Bartfai, G., Corona, G., Forti, G., Toppari, J., Goulis, D. G., & Jorgensen, N. (2019). EAA clinical practice guidelines-gynecomastia evaluation and management. Andrology, 7(6), 778-793. https://doi.org/10.1111/andr.12636
- Khashchenko, E., Uvarova, E., Vysokikh, M., Ivanets, T., Krechetova, L., Tarasova, N., Sukhanova, I., Mamedova, F., Borovikov, P., Balashov, I., & Sukhikh, G. (2020). The Relevant Hormonal Levels and Diagnostic Features of Polycystic Ovary Syndrome in Adolescents. J Clin Med, 9(6). https://doi.org/10.3390/jcm9061831
- Kinter, K. J., & Anekar, A. A. (2020). Biochemistry, Dihydrotestosterone. StatPearls Publishing, Treasure Island (FL). https://www.ncbi.nlm.nih.gov/books/NBK557634
- Legro, R. S., Arslanian, S. A., Ehrmann, D. A., Hoeger, K. M., Murad, M. H., Pasquali, R., & Welt, C. K. (2013). Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab, 98(12), 4565-4592. https://doi.org/10.1210/jc.2013-2350
- Longcope, C. (1986). Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab, 15(2), 213-228. https://doi.org/10.1016/s0300-595x(86)80021-4
- Lynch, K. L. (2016). CLSI C62-A: A New Standard for Clinical Mass Spectrometry. Clinical Chemistry, 62(1), 24-29. https://doi.org/10.1373/clinchem.2015.238626
- Maimoun, L., Philibert, P., Cammas, B., Audran, F., Bouchard, P., Fenichel, P., Cartigny, M., Pienkowski, C., Polak, M., Skordis, N., Mazen, I., Ocal, G., Berberoglu, M., Reynaud, R., Baumann, C., Cabrol, S., Simon, D., Kayemba-Kay's, K., De Kerdanet, M., . . . Sultan, C. (2011). Phenotypical, biological, and molecular heterogeneity of 5α-reductase deficiency: an extensive international experience of 55 patients. J Clin Endocrinol Metab, 96(2), 296-307. https://doi.org/10.1210/jc.2010-1024
- Meldrum, D. R., Gambone, J. C., Morris, M. A., Esposito, K., Giugliano, D., & Ignarro, L. J. (2012). Lifestyle and metabolic approaches to maximizing erectile and vascular health. Int J Impot Res, 24(2), 61-68. https://doi.org/10.1038/ijir.2011.51
- Mezzullo, M., Fazzini, A., Gambineri, A., Di Dalmazi, G., Mazza, R., Pelusi, C., Vicennati, V., Pasquali, R., Pagotto, U., & Fanelli, F. (2017). Parallel diurnal fluctuation of testosterone, androstenedione, dehydroepiandrosterone and 17OHprogesterone as assessed in serum and saliva: validation of a novel liquid chromatography-tandem mass spectrometry method for salivary steroid profiling. Clin Chem Lab Med, 55(9), 1315-1323. https://doi.org/10.1515/cclm-2016-0805
- Mohammed, M., Al-Habori, M., Abdullateef, A., & Saif-Ali, R. (2018). Impact of Metabolic Syndrome Factors on Testosterone and SHBG in Type 2 Diabetes Mellitus and Metabolic Syndrome. J Diabetes Res, 2018, 4926789. https://doi.org/10.1155/2018/4926789
- Molina-Vega, M., Munoz-Garach, A., Damas-Fuentes, M., Fernandez-Garcia, J. C., & Tinahones, F. J. (2018). Secondary male hypogonadism: A prevalent but overlooked comorbidity of obesity. Asian J Androl. https://doi.org/10.4103/aja.aja_44_18
- Morales, A., Bebb, R. A., Manjoo, P., Assimakopoulos, P., Axler, J., Collier, C., Elliott, S., Goldenberg, L., Gottesman, I., Grober, E. D., Guyatt, G. H., Holmes, D. T., & Lee, J. C. (2015). Diagnosis and management of testosterone deficiency syndrome in men: clinical practice guideline. Cmaj, 187(18), 1369-1377. https://doi.org/10.1503/cmaj.150033
- Mulhall, J. P., Trost, L. W., Brannigan, R. E., Kurtz, E. G., Redmon, J. B., Chiles, K. A., Lightner, D. J., Miner, M. M., Murad, M. H., Nelson, C. J., Platz, E. A., Ramanathan, L. V., & Lewis, R. W. (2014, 2018). Evaluation and Management of Testosterone Deficiency. American Urological Association. Retrieved 10/22/2018 from https://www.auanet.org/guidelines-and-quality/guidelines/testosterone-deficiency-guideline
- Nassar, G. N., & Leslie, S. W. (2023). Physiology, Testosterone. In StatPearls. StatPearls Publishing LLC. https://www.ncbi.nlm.nih.gov/books/NBK526128/
- NCCN. (2024a). Neuroendocrine and Adrenal Tumors Version 2. 2024. https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- NCCN. (2024b). Prostate Cancer, Version 4, 2024. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- Rochira, V., Antonio, L., & Vanderschueren, D. (2018). EAA clinical guideline on management of bone health in the andrological outpatient clinic. Andrology, 6(2), 272-285. https://doi.org/10.1111/andr.12470
- Sachdev, S., Cucchiara, A. J., & Snyder, P. J. (2020). Prostate Specific Antigen Concentrations in Response to Testosterone Treatment of Severely Hypogonadal Men. Journal of the Endocrine Society. https://doi.org/10.1210/jendso/bvaa141
- Salonia, Bettocchi, Carvalho, Corona, Jones, Kadioglu, Martinez-Salamanca, Minhas, Serefoǧlu, & Verze. (2024). European Association of Urology: Sexual and Reproductive Health. https://uroweb.org/guidelines/sexual-and-reproductive-health
- Sartorius, G., Ly, L. P., Sikaris, K., McLachlan, R., & Handelsman, D. J. (2009). Predictive accuracy and sources of variability in calculated free testosterone estimates. Ann Clin Biochem, 46(Pt 2), 137-143. https://doi.org/10.1258/acb.2008.008171
- Schulster, M., Bernie, A. M., & Ramasamy, R. (2016). The role of estradiol in male reproductive function. Asian J Androl, 18(3), 435-440. https://doi.org/10.4103/1008-682x.173932
- Shukla, A., Sharda, B., Bhardwaj, S., Kailash, U., Kalani, R., Satyanarayana, L., & Shrivastava, A. (2018). Association Between Serum Testosterone and Serum PSA Among Men With and Without Partial Androgen Deficiency. Indian Journal of Clinical Biochemistry, 1-5. https://link.springer.com/article/10.1007/s12291-018-0785-3
- Skinner, R., Mulder, R. L., Kremer, L. C., Hudson, M. M., Constine, L. S., Bardi, E., Boekhout, A., Borgmann-Staudt, A., Brown, M. C., Cohn, R., Dirksen, U., Giwercman, A., Ishiguro, H., Jahnukainen, K., Kenney, L. B., Loonen, J. J., Meacham, L., Neggers, S., Nussey, S., . . . Green, D. M. (2017). Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol, 18(2), e75-e90. https://doi.org/10.1016/s1470-2045(17)30026-8
- Snyder, P. J. (2024). Clinical features and diagnosis of male hypogonadism. Wolters Kluwer. https://www.uptodate.com/contents/clinical-features-and-diagnosis-of-male-hypogonadism
- Stanworth, R. D., & Jones, T. H. (2008). Testosterone for the aging male; current evidence and recommended practice. Clin Interv Aging, 3(1), 25-44. https://doi.org/10.2147/cia.s190
- Star-Weinstock, M., & Dey, S. (2019). Development of a CDC-certified total testosterone assay for adult and pediatric samples using LC–MS/MS. Clinical Mass Spectrometry, 13, 27-35. https://www.sciencedirect.com/science/article/pii/S2376999819300017
- Stern, J., & Casto, K. (2024). Salivary testosterone across the menstrual cycle. Horm Behav, 164, 105608. https://doi.org/10.1016/j.yhbeh.2024.105608
- Sun, G., Xue, J., Li, L., Li, X., Cui, Y., Qiao, B., Wei, D., & Li, H. (2020). Quantitative determination of human serum testosterone via isotope dilution ultra‑performance liquid chromatography tandem mass spectrometry. Mol Med Rep, 22(2), 1576-1582. https://doi.org/10.3892/mmr.2020.11235
- Teede, H. J., Misso, M. L., Costello, M. F., Dokras, A., Laven, J., Moran, L., Piltonen, T., & Norman, R. J. (2018). Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod, 33(9), 1602-1618. https://doi.org/10.1093/humrep/dey256
- Travison, T. G., Vesper, H. W., Orwoll, E., Wu, F., Kaufman, J. M., Wang, Y., Lapauw, B., Fiers, T., Matsumoto, A. M., & Bhasin, S. (2017). Harmonized Reference Ranges for Circulating Testosterone Levels in Men of Four Cohort Studies in the United States and Europe. J Clin Endocrinol Metab, 102(4), 1161-1173. https://doi.org/10.1210/jc.2016-2935
- van der Veen, A., van Faassen, M., de Jong, W. H. A., van Beek, A. P., Dijck-Brouwer, D. A. J., & Kema, I. P. (2019). Development and validation of a LC-MS/MS method for the establishment of reference intervals and biological variation for five plasma steroid hormones. Clin Biochem, 68, 15-23. https://doi.org/10.1016/j.clinbiochem.2019.03.013
- Vermeulen, A., Verdonck, L., & Kaufman, J. M. (1999). A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab, 84(10), 3666-3672. https://doi.org/10.1210/jcem.84.10.6079
- Vesper, H., Botelho, J., & Wang, Y. (2014). Challenges and improvements in testosterone and estradiol testing [Invited Review]. Asian Journal of Andrology, 16(2), 178-184. https://doi.org/10.4103/1008-682x.122338
- Viana, A., Jr., Daflon, A. C., Couto, A., Neves, D., de Araujo-Melo, M. H., & Capasso, R. (2017). Nocturnal Hypoxemia is Associated With Low Testosterone Levels in Overweight Males and Older Men With Normal Weight. J Clin Sleep Med, 13(12), 1395-1401. https://doi.org/10.5664/jcsm.6832
- Wang, A., Arver, S., Flanagan, J., Gyberg, V., Nasman, P., Ritsinger, V., & Mellbin, L. G. (2018). Dynamics of testosterone levels in patients with newly detected glucose abnormalities and acute myocardial infarction. Diab Vasc Dis Res, 1479164118802543. https://doi.org/10.1177/1479164118802543
- Welker, K. M., Lassetter, B., Brandes, C. M., Prasad, S., Koop, D. R., & Mehta, P. H. (2016). A comparison of salivary testosterone measurement using immunoassays and tandem mass spectrometry. Psychoneuroendocrinology, 71, 180-188. https://doi.org/10.1016/j.psyneuen.2016.05.022
- Yun, Y.-M., Botelho, J. C., Chandler, D. W., Katayev, A., Roberts, W. L., Stanczyk, F. Z., Vesper, H. W., Nakamoto, J. M., Garibaldi, L., Clarke, N. J., & Fitzgerald, R. L. (2012). Performance Criteria for Testosterone Measurements Based on Biological Variation in Adult Males: Recommendations from the Partnership for the Accurate Testing of Hormones. Clinical Chemistry, 58(12), 1703-1710. https://doi.org/10.1373/clinchem.2012.186569
- Zakharov, M. N., Bhasin, S., Travison, T. G., Xue, R., Ulloor, J., Vasan, R. S., Carter, E., Wu, F., & Jasuja, R. (2015). A multi-step, dynamic allosteric model of testosterone's binding to sex hormone binding globulin. Mol Cell Endocrinol, 399, 190-200. https://doi.org/10.1016/j.mce.2014.09.001
- Zitzmann, M., Nieschlag, E., Traish, A., & Kliesch, S. (2019). Testosterone Treatment in Men with Classical vs. Functional Hypogonadism: A 9-Year Registry. Journal of the Endocrine Society, 3. https://doi.org/10.1210/js.2019-SUN-222
Coding Section
| Codes | Number | Description |
| CPT | 82040 | Albumin; serum, plasma or whole blood |
| 82642 (effective 01/01/2019) | Dihydrotestosterone (DHT) | |
| 82670 | Estradiol; total | |
| 82681 (effective 01/01/2021) | Estradiol; free, direct measurement (e.g., equilibrium dialysis) | |
| 84270 | Sex hormone binding globulin (SHBG) | |
| 84402 | Testosterone; free | |
| 84403 | Testosterone; total | |
| 84410 (effective 1/1/2017) | Testosterone; bioavailable, direct measurement (e.g., differential precipitation) | |
| ICD-10-CM | E28.1 | Androgen excess |
| E29.0-E29.9 | Testicular dysfunction | |
| E30.0 | Delayed puberty | |
| F32.0-F33.9 | Depression |
|
| G47.8-G47.9 | Sleep disorders | |
| N50.0 | Atrophy of testis | |
| N50.8 | Other spec disorders, male genital organs | |
| N50.9 | Disorder of male genital organs, unspecified | |
| N64.4 | Breast pain | |
| R41.840 | Attention/concentration deficit | |
| R41.9 | Unspecified symptoms/signs involving cognitive functions and awareness | |
| R53.81, R53.83 | Fatigue |
|
| R68.82 | Decreased libido | |
| Z92.240-Z92.241 | Personal history, steroid therapy | |
| L70.0-L70.9 | Acne | |
| R63.5 | Excessive weight gain | |
| L68.0 | Hirsutism | |
| N91.0-N91.5 | Absent, scanty and rare menstruation | |
| N92.0-N92.6 | Excessive, frequent/irregular menstruation | |
| N94.89-N94.9 | Specified conditions associated with female genital organs and menstrual cycle | |
| N97.0-N97.9 | Female infertility | |
| E88.81 | Metabolic syndrome (insulin resistance) | |
| R73.01-R73.09 | Abnormal glucose | |
| E08-E13 | Diabetes | |
| E28.2 | Polycystic Ovarian Syndrome | |
| E24.0-E24.9 | Cushing’s Syndrome | |
| E03.9 | Hypothyroidism | |
| E22.0 | Acromegaly | |
| E05.0-E05.9 | Thyrotoxicosis |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2016 Forward
| 01/15/2025 | Annual review, no change to policy intent. Updating table of terminology, rationale, references, and coding. |
| 01/23/2024 | Annual review, no change to policy intent. Updating Rationale and References. |
| 01/31/2023 | Annual review, no change to policy intent, but, policy updated for clarity and consistency. Note/guideline 1 updated regarding timing for testing. Also updating description, rationale and references. |
| 01/31/2022 |
Annual review, updating policy verbiage for clarity and adding medical necessity regardidng testing for androgen deficiency for members who are/were female at birth. No other changes. |
| 01/12/2021 |
Annual review, no change to policy intent. |
| 12/10/2020 |
Updated Coding Section with 2021 codes. |
| 01/02/2020 |
Annual review, no change to policy intent |
| 02/05/2019 |
Annual review. Updating policy. |
| 01/07/2019 |
Added effective date to code 82642 |
| 12/19/2019 |
Updating with 2019 codes. |
| 01/30/2018 |
Annual review, no change to policy intent |
| 04/26/2017 |
Updated category to Laboratory. No other changes made. |
| 04/10/2017 |
Interim Review. Updated policy verbiage. No other changes made. |
| 1/25/2017 |
Addition to annual review. Adding an example of what androgen excess in females would be and updating the verbiage in criteria number 7 to include enzyme inhibitors. |
| 01/03/2017 |
Annual review, no change to policy intent. |
| 11/30/2016 |
Updated Coding Section with 2017 codes. |
| 01/07/2016 |
NEW POLICY |